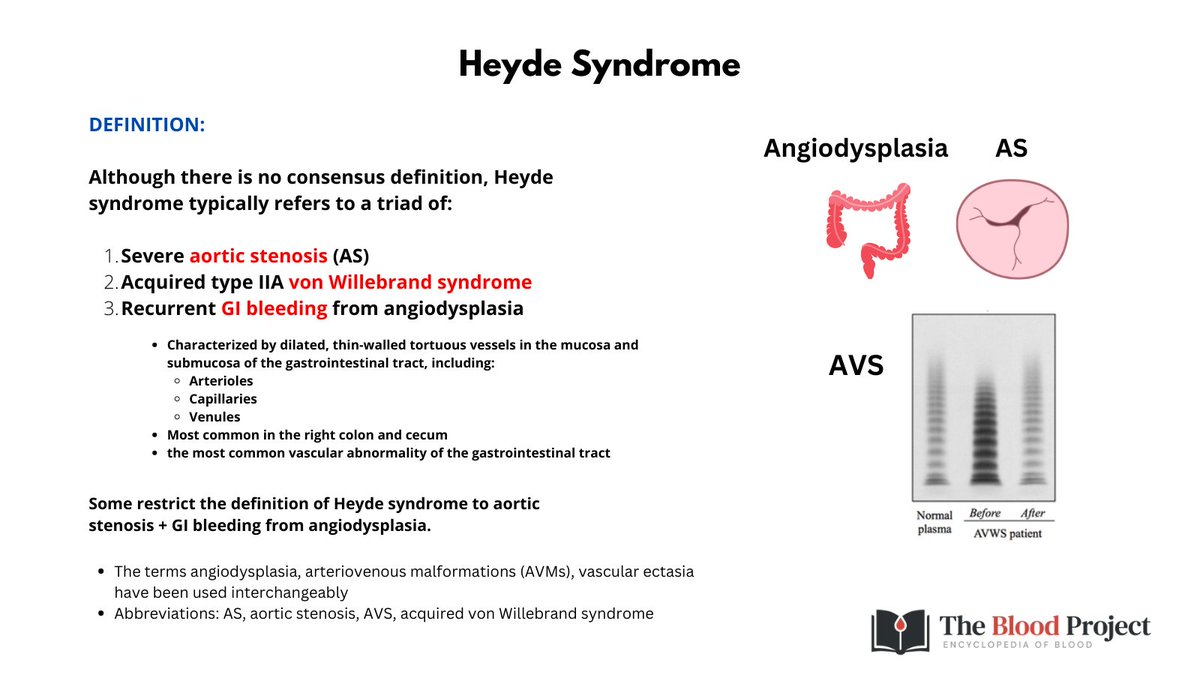
1/9
@jlberrymd #b12deficiency
Here's a cool case with an important take-home message.
A 48 yo M is referred to you with macrocytic anemia. Before looking at the graphic you should have a rough differential diagnosis at your finger tips!
@jlberrymd #b12deficiency
Here's a cool case with an important take-home message.
A 48 yo M is referred to you with macrocytic anemia. Before looking at the graphic you should have a rough differential diagnosis at your finger tips!

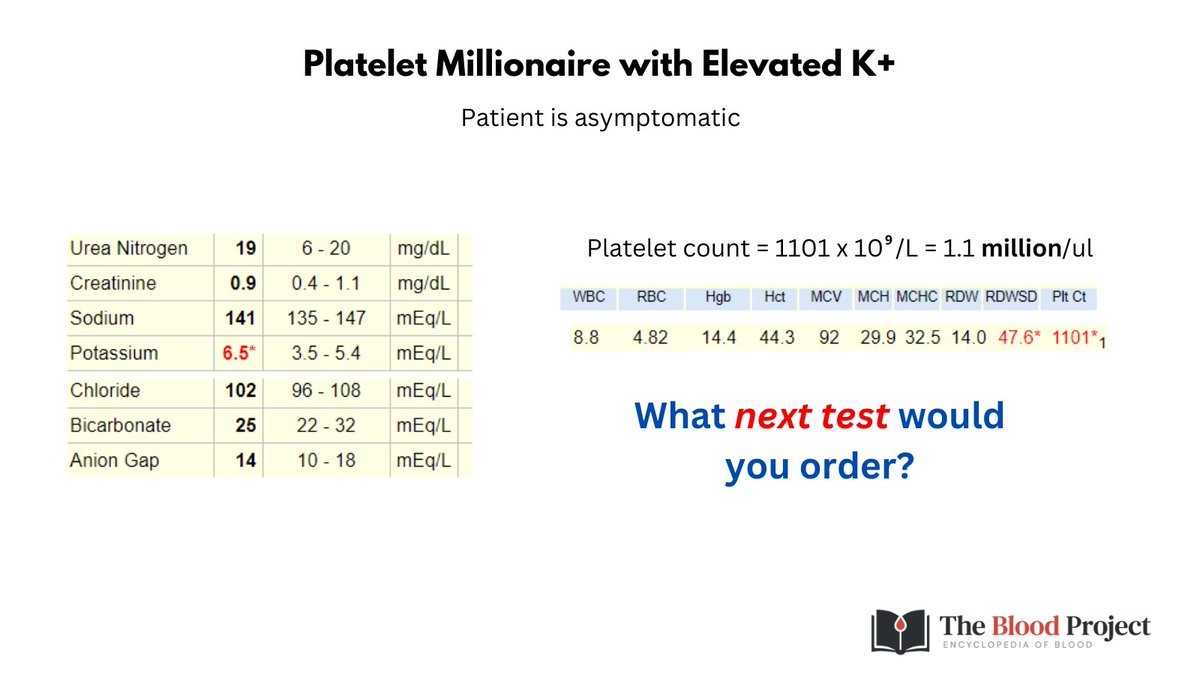
2/9
Peripheral smear shows large RBCs, anisocytosis but otherwise unremarkable.
You work up the patient for macrocytic anemia by checking B12/folate and copper levels, liver function, thyroid function, SPEP but you stop short of a bone marrow biopsy. See graphic for results.
Peripheral smear shows large RBCs, anisocytosis but otherwise unremarkable.
You work up the patient for macrocytic anemia by checking B12/folate and copper levels, liver function, thyroid function, SPEP but you stop short of a bone marrow biopsy. See graphic for results.

3/9
All lab tests are negative with the exception of a (VERY) elevated LDH, an increased AST:ALT ratio and low haptoglobin. A CT abdomen was normal (as was the PT/INR and serum albumin).
What is this suggestive of?
All lab tests are negative with the exception of a (VERY) elevated LDH, an increased AST:ALT ratio and low haptoglobin. A CT abdomen was normal (as was the PT/INR and serum albumin).
What is this suggestive of?
4/9
The results are suggestive of hemolysis.
Yet, the retics are low.
... and the LDH is disproportionately high.
High suspicion for ineffective erythropoiesis secondary to B12 deficiency!
The results are suggestive of hemolysis.
Yet, the retics are low.
... and the LDH is disproportionately high.
High suspicion for ineffective erythropoiesis secondary to B12 deficiency!
5/9
OK, now a little Hx and Px 😉:
On Hx, the patient complained of fatigue and shortness of breath on exertion. There was no past history of abdominal surgery. He was not a vegetarian. Px exam revealed glossitis. CNS status was normal.
OK, now a little Hx and Px 😉:
On Hx, the patient complained of fatigue and shortness of breath on exertion. There was no past history of abdominal surgery. He was not a vegetarian. Px exam revealed glossitis. CNS status was normal.

6/9
Serum B12 levels are NOT 100% sensitive for diagnosing B12 deficiency. If clinical suspicion is high, the next step is to check for functional B12 deficiency by measuring serum MMA and HCY (per BSH guidelines [and common sense]).
Serum B12 levels are NOT 100% sensitive for diagnosing B12 deficiency. If clinical suspicion is high, the next step is to check for functional B12 deficiency by measuring serum MMA and HCY (per BSH guidelines [and common sense]).

7/9
The MMA (more specific than HCY) was astronomically high, clinching the diagnosis of functional B12 deficiency. Anti-intrinsic factor antibodies were positive, c/w a diagnosis of pernicious anemia. The patient received B12 therapy and his labs values normalized.
The MMA (more specific than HCY) was astronomically high, clinching the diagnosis of functional B12 deficiency. Anti-intrinsic factor antibodies were positive, c/w a diagnosis of pernicious anemia. The patient received B12 therapy and his labs values normalized.

8/9
It is not clear why some patients with functional/cellular B12 deficiency have normal serum B12 levels.
In most cases, it is likely related to increased binding of B12 to haptocorrin at the expense of transcobalamin II (the functional B12 transport protein).
It is not clear why some patients with functional/cellular B12 deficiency have normal serum B12 levels.
In most cases, it is likely related to increased binding of B12 to haptocorrin at the expense of transcobalamin II (the functional B12 transport protein).
9/9
In any event, the take home message is:
DO NOT DISCOUNT B12 DEFICIENCY BECAUSE THE SERUM B12 LEVEL IS NORMAL!!!
For infographic on B12 deficiency, see:
thebloodproject.com/copy-pa/
For case study of B12 deficiency, see:
thebloodproject.com/cases-archive/…
In any event, the take home message is:
DO NOT DISCOUNT B12 DEFICIENCY BECAUSE THE SERUM B12 LEVEL IS NORMAL!!!
For infographic on B12 deficiency, see:
thebloodproject.com/copy-pa/
For case study of B12 deficiency, see:
thebloodproject.com/cases-archive/…
• • •
Missing some Tweet in this thread? You can try to
force a refresh