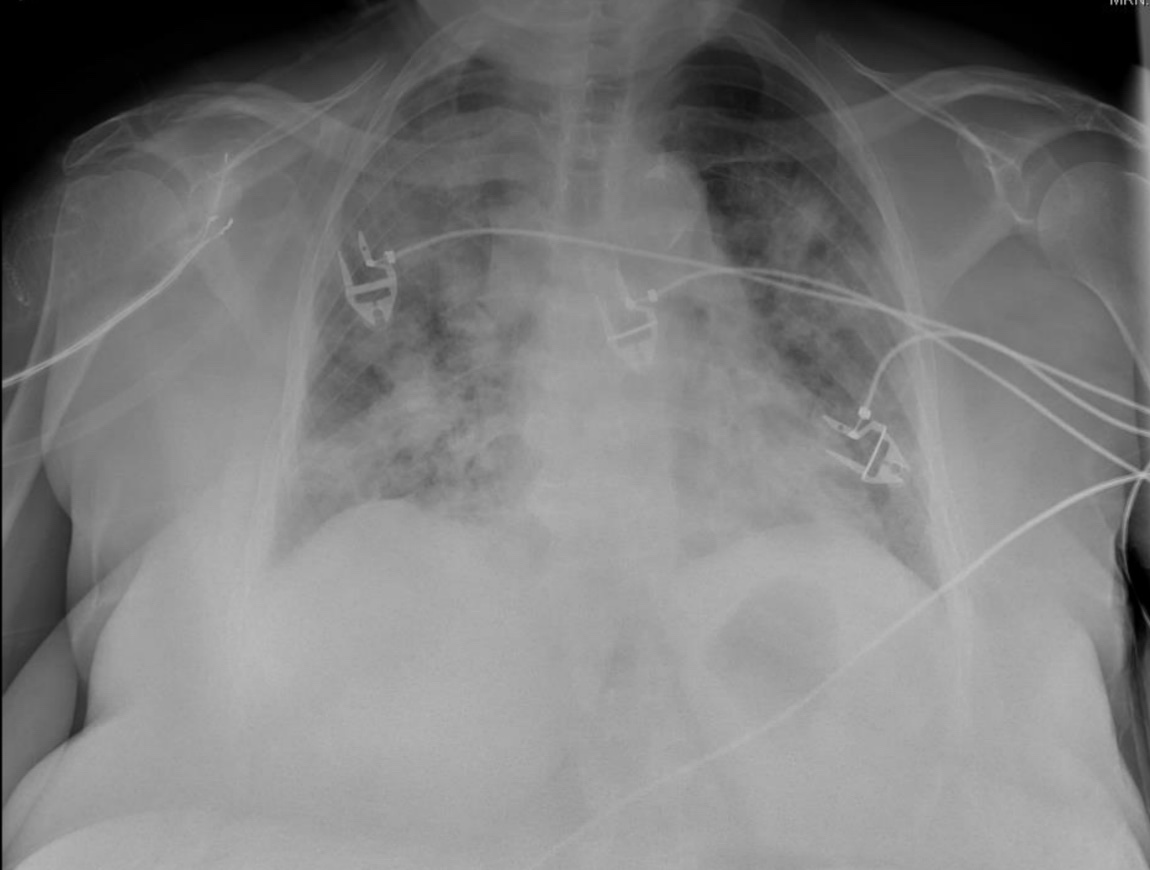
Here’s a critical care puzzle & illustrates some important cardiopulmonary physiology:
These two pictures were taken just an hour apart. What intervention was done in between that changed the respiratory pattern? (Red box)
Multiple choice & answers in the 🧵.
1/

These two pictures were taken just an hour apart. What intervention was done in between that changed the respiratory pattern? (Red box)
Multiple choice & answers in the 🧵.
1/

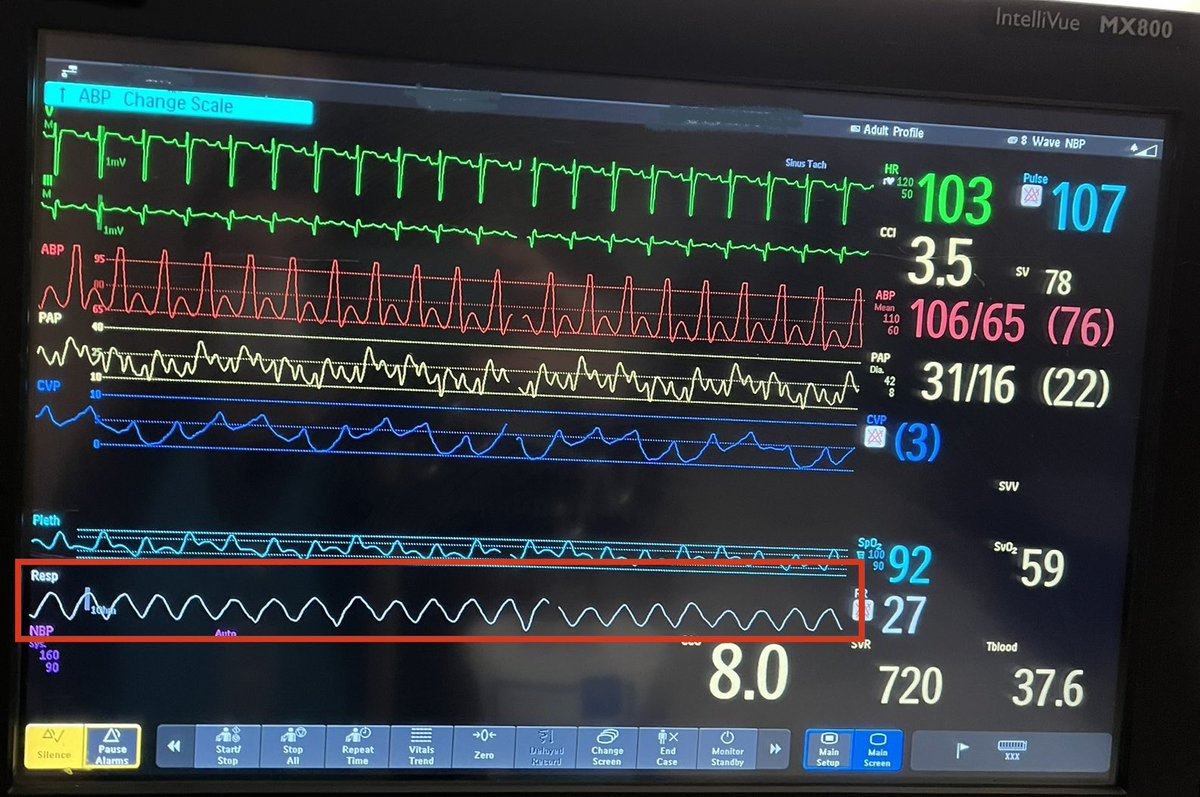
The intervention was:
2/
2/
This is Cheyne-Stokes Respirations (CSR) in a person with heart failure.
The intervention was dobutamine (an inotrope).
Cheyne-Stokes is a characteristically regular crescendo-descresendo respiratory pattern with interspersed periods of apnea.
3/


The intervention was dobutamine (an inotrope).
Cheyne-Stokes is a characteristically regular crescendo-descresendo respiratory pattern with interspersed periods of apnea.
3/



But *WHY* do people with heart failure develop CSR?
And *WHY* does an inotrope help?
To answer this we need to understand control of respiration. As a bonus we'll learn about control theory & how your thermostat works!
Buckle up for a #physiology #engineering #tweetorial!
4/


And *WHY* does an inotrope help?
To answer this we need to understand control of respiration. As a bonus we'll learn about control theory & how your thermostat works!
Buckle up for a #physiology #engineering #tweetorial!
4/


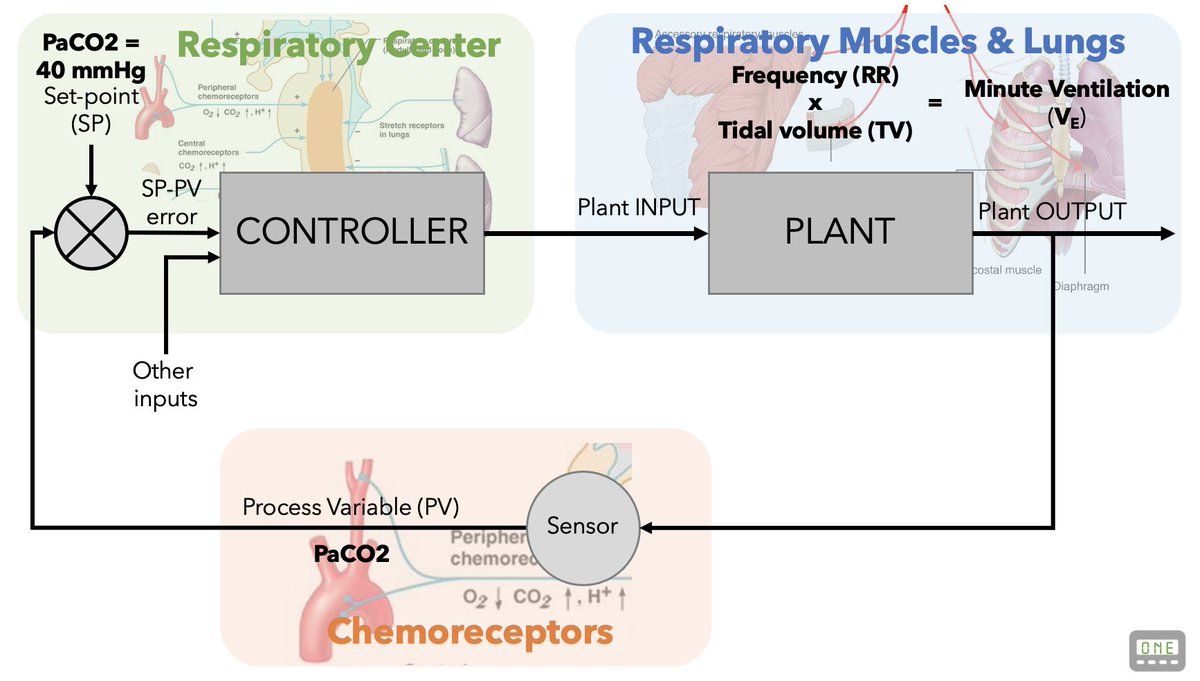
We know conceptually that PaCO2 (also pH & PaO2) stimulates respiratory drive & minute ventilation (VE)
Chemoreceptors sense PaCO2 & trigger respiratory centers in the pons/medulla. These activate the respiratory muscles & trigger breathing.
But HOW does this actually work?
5/
Chemoreceptors sense PaCO2 & trigger respiratory centers in the pons/medulla. These activate the respiratory muscles & trigger breathing.
But HOW does this actually work?
5/

But even though we understand the parts of the respiratory system, we need a way to understand its *dynamics*
There's a field of engineering called Control Theory that allows us to accurately model complex dynamical systems.
en.wikipedia.org/wiki/Control_t…
6/
There's a field of engineering called Control Theory that allows us to accurately model complex dynamical systems.
en.wikipedia.org/wiki/Control_t…
6/

Let's take a step back and introduce an analogy: imagine a home with a thermostat and a radiator.
When the temperature drops below a set-point, the THERMOSTAT turns the RADIATOR on, increasing the temperature. When the desired temperature is reached it turns off.
7/

When the temperature drops below a set-point, the THERMOSTAT turns the RADIATOR on, increasing the temperature. When the desired temperature is reached it turns off.
7/


This is an example of a controlled system: a CONTROLLER (the thermostat) directs a PLANT (the radiator) to regulate a process variable (the temperature).
8/
8/

This closed loop system carefully regulates the temperature in our homes.
We'll call this HOMEostasis...! 😂
9/
We'll call this HOMEostasis...! 😂
9/
It also turns out this simple Control Theory Model is also a pretty good analogy of how our respiratory system functions:
A CONTROLLER (the pons/medulla) activates a PLANT (the respiratory muscles) in response to a PROCESS VARIABLE (PaCO2).
10/

A CONTROLLER (the pons/medulla) activates a PLANT (the respiratory muscles) in response to a PROCESS VARIABLE (PaCO2).
10/


Just like our home thermostat regulates temperature, our pons/medulla activates our respiratory muscles using a closed loop controlled system.
Normally, this adjusts VE to maintain homeostasis, tightly controlling our PaCO2, PaO2 & pH.
en.wikipedia.org/wiki/Cheyne%E2…
11/
Normally, this adjusts VE to maintain homeostasis, tightly controlling our PaCO2, PaO2 & pH.
en.wikipedia.org/wiki/Cheyne%E2…
11/

Full disclosure: As you can see, I've simplified the model & omitted the math (this is a #tweetorial not a textbook!).
If I've piqued your interest in the topic I recommend reading this paper (don't worry you won't have to do any Laplace transforms!)
jstage.jst.go.jp/article/jpfsm/…
12/


If I've piqued your interest in the topic I recommend reading this paper (don't worry you won't have to do any Laplace transforms!)
jstage.jst.go.jp/article/jpfsm/…
12/



Now that we understand how the system works, we're ready to understand how it's perturbed in CHF.
Using our analogy:
1️⃣weaker radiator
2️⃣radiator is farther from the thermostat
These result in delayed response to temperature shifts & thus big swings in room temperature.
13/



Using our analogy:
1️⃣weaker radiator
2️⃣radiator is farther from the thermostat
These result in delayed response to temperature shifts & thus big swings in room temperature.
13/




Why is the radiator smaller?
Because of low cardiac output, less blood is delivered to the lungs. This increases physiologic DEAD SPACE & alters the relationship between VE and PaCO2.
In Control Theory this is called a change in "PLANT GAIN" (PG)
ahajournals.org/doi/10.1161/JA…
14/

Because of low cardiac output, less blood is delivered to the lungs. This increases physiologic DEAD SPACE & alters the relationship between VE and PaCO2.
In Control Theory this is called a change in "PLANT GAIN" (PG)
ahajournals.org/doi/10.1161/JA…
14/


Why is the radiator farther away?
Due to low cardiac output, it takes longer for blood to circulate from lungs to chemoreceptors. This means that there is a DELAY (circulation time) between plant output and sensor.
15/
Due to low cardiac output, it takes longer for blood to circulate from lungs to chemoreceptors. This means that there is a DELAY (circulation time) between plant output and sensor.
15/

How much longer is circulation time in CHF?
In 1933, researchers injected volunteers in the leg with a tracer compound and measured how many seconds until the volunteers could taste it.
Normal circulating time: 13 sec (range 10-16)
CHF circulating time: 26 sec (range 17-47)
16/

In 1933, researchers injected volunteers in the leg with a tracer compound and measured how many seconds until the volunteers could taste it.
Normal circulating time: 13 sec (range 10-16)
CHF circulating time: 26 sec (range 17-47)
16/


🚨 Clinical aside: This fact can save a life!
Increased circulating time really matters when you intubate people with CHF:
-Expect your sedation & paralytics to take longer to work!
-There will be a longer delay in SpO2 recovery once the tube is in!
Be patient!
17/
Increased circulating time really matters when you intubate people with CHF:
-Expect your sedation & paralytics to take longer to work!
-There will be a longer delay in SpO2 recovery once the tube is in!
Be patient!
17/
Adding a delay between plant output & controller input can destabilize a controlled system.
For the mathematically inclined, adding a time delay (τ) has an *exponential* effect on the Lapacian. This is why a small delay (just 13 seconds) can profoundly destabilize things!
18/
For the mathematically inclined, adding a time delay (τ) has an *exponential* effect on the Lapacian. This is why a small delay (just 13 seconds) can profoundly destabilize things!
18/

Altered plant gain & prolonged circulating time can make feedback loops overcorrect; VE is constantly overshooting (hyperventilation) or undershooting (apnea).
Each correction leads to another cycle of larger corrections, until large oscillations develop: Cheyne-Stokes!
19/


Each correction leads to another cycle of larger corrections, until large oscillations develop: Cheyne-Stokes!
19/



Let's summarize:
- the respiratory "plant" is triggered by the medulla/pons "controller"
- people with CHF have more dead space (a smaller plant) & delay in sensing CO2; this causes Periodic instability in PaCO2 and respirations!
- think of the thermostat overcorrecting!
20/
- the respiratory "plant" is triggered by the medulla/pons "controller"
- people with CHF have more dead space (a smaller plant) & delay in sensing CO2; this causes Periodic instability in PaCO2 and respirations!
- think of the thermostat overcorrecting!
20/
But why did an inotrope "fix" the Cheyne-Stokes respirations?
- the inotrope increased the SV & CI
- this reduced physiologic dead space, making the lungs work better (improved plant gain!)
- this also reduced circulating time (eliminating the instability from the delay!)
21/

- the inotrope increased the SV & CI
- this reduced physiologic dead space, making the lungs work better (improved plant gain!)
- this also reduced circulating time (eliminating the instability from the delay!)
21/


If you like my thermostat analogy, imagine that adding an inotrope is like putting a fan in that big room!
The fan improves the efficiency of the radiator & reduces the delay in sensing. This "fixes" the problem of big swings in temperature, restoring HOMEostasis!
22/
The fan improves the efficiency of the radiator & reduces the delay in sensing. This "fixes" the problem of big swings in temperature, restoring HOMEostasis!
22/
Let's go over the incorrect answers.
- Opioids exacerbate Cheyne-Stokes (CSR)
- Oxygen can help CSR but wouldn't have doubled the SV or CO!
- This was CSR not Kussmaul. If it was Kussmaul due to DKA, insulin would have helped.
23/
- Opioids exacerbate Cheyne-Stokes (CSR)
- Oxygen can help CSR but wouldn't have doubled the SV or CO!
- This was CSR not Kussmaul. If it was Kussmaul due to DKA, insulin would have helped.
23/

To summarize everything, we learned:
- how control theory helps us understand control of respiration (thermostat analogy)
- why people with CHF develop Cheyne-Stokes: more dead space & prolonged circulatory time (big room, small radiator)
- how inotropes correct CSR (add a fan)



- how control theory helps us understand control of respiration (thermostat analogy)
- why people with CHF develop Cheyne-Stokes: more dead space & prolonged circulatory time (big room, small radiator)
- how inotropes correct CSR (add a fan)




It occurs to me that a slightly better analogy would be a thermostat turning on central AC:
Rising temp (analogous to PaCO2) leads to AC plant activation (analogous to ventilation), which normalizes the temp!
🥶But frankly it’s way too cold out to think about AC!
Rising temp (analogous to PaCO2) leads to AC plant activation (analogous to ventilation), which normalizes the temp!
🥶But frankly it’s way too cold out to think about AC!
• • •
Missing some Tweet in this thread? You can try to
force a refresh