
Big @batsgoviral week! In @PNASNews and @ScienceAdvances we test how to prevent zoonotic #spillover by interrupting virus transmission in bat reservoirs. Short story: culls didn’t reduce spillover but altered rabies spatial spread; transmissible vaccines may be a solution. A 🧵. 
First culling. Vampire bats are poisoned throughout Latin America to reduce blood feeding on humans and livestock and in hopes of preventing uniformly lethal rabies infections. Whether culls reduce rabies spillover is still controversial even after ~50 years of use. 

We studied a 2 year bat cull in southern Peru where >21,000 bats were treated with a spreadable poison #vampiricide. This region suffered >1000 rabies outbreaks from 2003-2019 making a rare & statistically powerful case study to test whether bat culls reduce viral spillover. 

Bayesian state space models by @MafaldaSViana & @themonkey_lab showed culls did not reduce rabies spillover to livestock, but marginally *increased* the chance of outbreaks in neighboring districts. 

Viral phylogenomics confirmed that culls altered viral spatial spread but revealed hidden complexity. Preventive culls delayed the arrival of viral lineages, but reactive culls (after outbreaks) accelerated virus spread elsewhere, a #perturbation effect of dispersing survivors 

For now, culling vampire bats remains inevitable despite its weak or counterproductive effects on rabies. Bat bites affect livestock health and productivity and attacks on humans must be controlled. But what next? 

That brings us to amazing work by @Megan3Griffiths which used 6 years of spatially replicated deep sequence data from a vampire bat betaherpesvirus to parameterize epidemiological models and test how a still-hypothetical vaccine might fare against rabies. 

A transmissible rabies vaccine with the epidemiological properties of the wildtype betaherpesvirus is predicted to vaccinate up to 80% of the vampire bat population with 50-95% reductions in rabies spillover risk…after release to just a single wild bat 
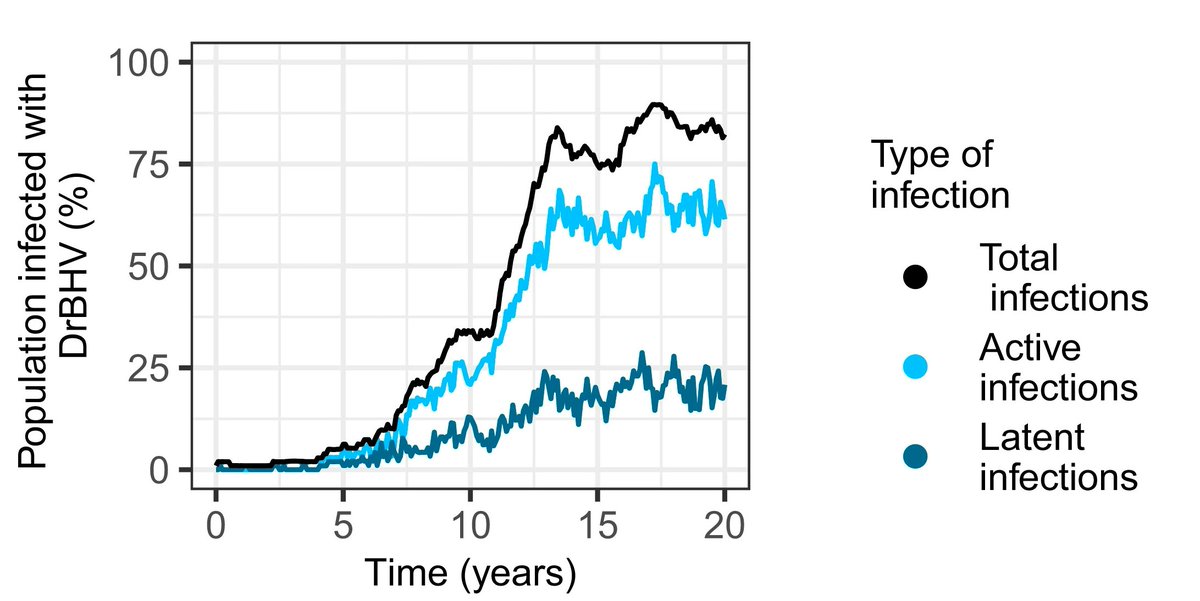
Even cooler, models show the herpesvirus persistently infects bats with cycles of latency and reactivation making a vaccine that is not just transmissible but also lifelong and #selfboosting. That potentially makes for a low-cost, high-impact and self-sustaining intervention 🏆
The papers can be found #OpenAccess here:
Transmissible vaccines ➡️ pnas.org/doi/10.1073/pn…
Culling ➡️ science.org/doi/10.1126/sc…
Funding from @wellcometrust & @The_MRC
Transmissible vaccines ➡️ pnas.org/doi/10.1073/pn…
Culling ➡️ science.org/doi/10.1126/sc…
Funding from @wellcometrust & @The_MRC
And some media coverage in @Nature by @JudeLB_Coleman ➡️ nature.com/articles/d4158…
&
@PopSci by @JoSolisM ➡️ popsci.com/environment/va…
(*do read past the headlines!)
&
@PopSci by @JoSolisM ➡️ popsci.com/environment/va…
(*do read past the headlines!)
• • •
Missing some Tweet in this thread? You can try to
force a refresh




