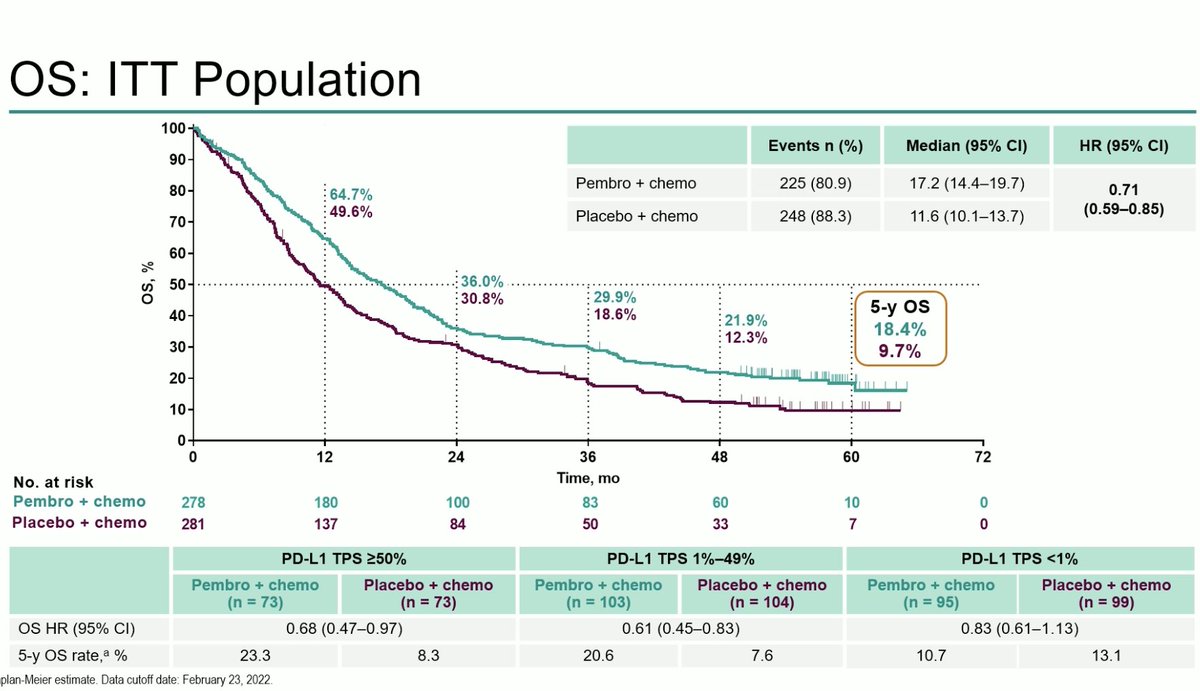
Impressive data from #AEGEAN at #AACR23 from Dr. John Heymach and colleagues. This is the first of several phase III peri-operative IO studies in resectable NSCLC combining neoadjuvant chemo-immunotherapy followed by adjuvant immunotherapy. 

Included stage IIA-IIIB (AJCC v8) with no EGFR/ALK & excluded pts who would require pneumonectomy. Large study with n=801 (CM816 was n=358). Pts received 4 cycles (not 3) of platinum-based chemo with durvalumab (anti-PDL1) or placebo, then surgery, then durvalumab / placebo x 1y. 

Almost 75% received carboplatin over cisplatin. Global study; fairly even PDL1 distribution. 80% of pts went to surgery, 78% completed resection (90-95% of those were R0). Overall, 66% of pts began adjuvant phase (but 90% of those who had an R0 resection had adjuvant therapy). 




Interim analysis of EFS from AEGEAN shows durvalumab pre/post surgery significantly improves PFS (HR 0.68) over placebo with 1y EFS rate 73.4% vs 64.5% and 2y EFS rate 63.3% vs 52.4% (EFS only 32% mature). Very early split of the curves with clear separation. #AACR23 

EFS subsets from AEGEAN show increased EFS with increasing PDL1 expression: HR 0.76 for PDL1 negative, HR 0.70 for PDL1 low, HR 0.60 for PDL1 high. KEYNOTE 091 remains outlier in perioperative space. EFS HR 0.95 for female pts but wide CI (hidden drivers? await further analysis). 



Neoadjuvant durvalumab over placebo increased pCR rate from 4.3% to 17.2% (+13%) and MPR from 12.3% to 33.3% (+21%). Comparable to CM816 (pCR 24%) and note larger sample size here and different methodology to define pCR. Awaiting outcomes by pCR status. #AACR23 
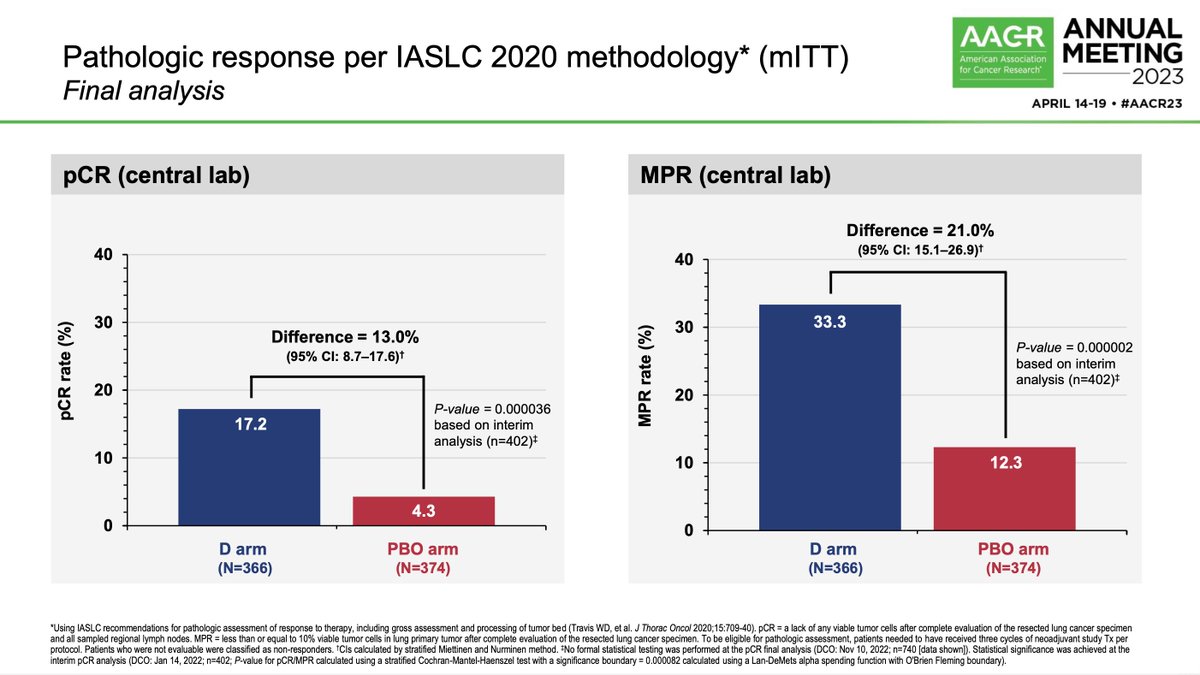
pCR also following expected PDL1 trends with 9% pCR in PDL1 negative (with durvalumab), 16% pCR in PDL1 low, 28% in PDL1 high. Higher pCR rate with carboplatin vs cisplatin (not powered for comparison but reassuring). Interesting pCR trends by stage and histology. #AACR23 

No concerning safety signals noted in AEGEAN. Rare to see AEs lead to cancellation of surgery (1-2%). G3+ immune mediated AEs in 4% with durvalumab and 2.5% with placebo. Many of the listed AEs expected with standard chemotherapy and surgery. #AACR23 



Overall, AEGEAN impressive: clear pCR benefit with addition of durvalumab to 4 cycles of chemotherapy and encouraging EFS rates. Contribution of adjuvant component remains unclear and won't be answered by this wave of studies but an immediate new standard once approved. #AACR23 

• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter