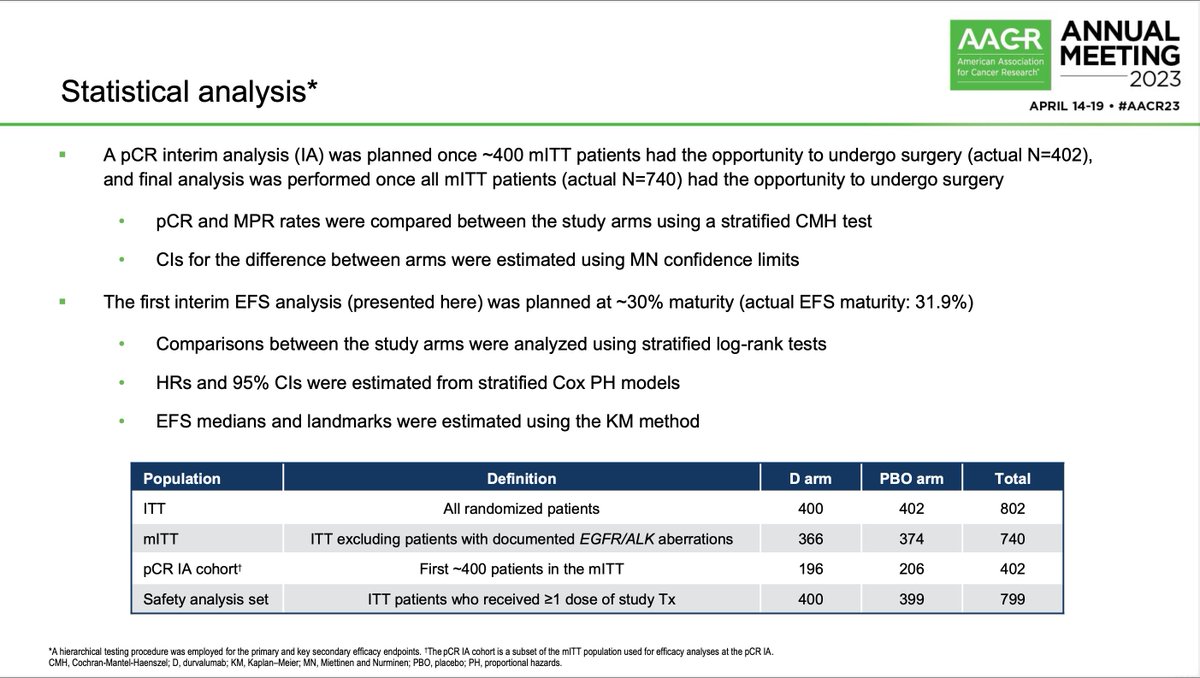
Dr. Shun Lu presents interim results from the perioperative NEOTORCH study at the #ASCOPlenarySeries. Randomized pts with resectable stage II/III NSCLC to perioperative chemotherapy with toripalimab (anti-PD1) vs placebo. Another entry in the perioperative space? #LCSM 

Includes resectable stage II/III (AJCC v8), EGFR/ALK wild type NSCLC. Pts receive 3 cycles of neoadjuvant chemotherapy + toripalimab vs placebo then surgery then 1 more cycle of adjuvant chemo (+tori/pbo) then 13 doses of maintenance toripalimab vs placebo. #ASCOPlenarySeries 

First interim EFS analysis only for stage III. Includes 404 pts - but 926 screened. Would be interesting to see specific eligibility criteria not met in screening process. Reminder of how selected trial populations are and value of future real-world analyses. #ASCOPlenarySeries 



NEOTORCH included 90% men, 78% squamous NSCLC. This is a far cry from AEGEAN (46% squamous) and CheckMate 816 (49% squamous). Includes 67% stage IIIA and 32% stage IIIB. 66% were PDL1 positive (no breakdown on high vs low yet). #ASCOPlenarySeries 

NEOTORCH interim results (for stage III NSCLC) show addition of perioperative toripalimab to chemotherapy improves event free survival with EFS HR 0.40 (same by IRC). Median EFS not reached for tori arm but 1y EFS rate 84% vs 57% and 2y EFS rate 65% vs 39%. Curves separate early. 

NEOTORCH subgroup analysis (for stage III) shows more benefit with adding toripalimab for PDL1+ subset (EFS HR 0.31 in PDL1+ and HR 0.59 in PDL1-) and squamous (this trial was mostly squamous, mostly male). #ASCOPlenarySeries 



NEOTORCH: Adding neoadjuvant toripalimab for resectable stage III NSCLC (mostly squamous) improves major pathologic response (MPR) rate from 8.4% to 48.5% and improves pathologic complete response (pCR) rate from 1% with chemotherapy to 28.2% with chemo-immunotherapy. #LCSM 



NEOTORCH survival analysis very immature but with 18m median follow up (for stage III), OS HR 0.62 with 2y OS rate 81% vs 74%. 

NEOTORCH: Among pts with resectable stage III NSCLC treated with neoadjuvant toripalimab + chemo x 3 cycles, 18% did not get to surgery (27% in placebo + chemo arm). Of the 82.2% that had surgery, 95.8% ha R0 resection. Similar to overall AEGEAN numbers. #ASCOPlenarySeries 

Note that 28% in the toripalimab + chemo arm had TEAEs leading to interruption (vs 14%) with 9% discontinuation (vs 7%) but most AEs were low grade and chemotherapy-related (myelosuppression). Higher rates of (low grade) thyroid and rash AEs and few with G3 pneumonitis. 







NEOTORCH shows that addition of anti-PD1 toripalimab to 3 cycles of neoadjuvant chemotherapy for resectable stage III NSCLC improves MPR and pCR rate and when coupled with 1 adjuvant cycle and 13 maintenance cycles, significantly improves EFS. #ASCOPlenarySeries 

• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter