Didn't have time to read the 150 pages of new information associated with @GOODMeat's FDA pre-market consultation for cultivated chicken?
Key info in this thread.
npr.org/sections/healt…
Key info in this thread.
npr.org/sections/healt…
Let's start with the conclusion. The FDA states, "foods comprised of, or containing, cultured chicken cell material resulting from the production process defined in CCC 000001 are as safe as comparable foods produced by other methods."
So how is it made?
So how is it made?
Cells: The cells used for production are a publicly available cell line known as DF-1, which has been in the ATCC cell bank since 1996. The cell line is a chicken fibroblast line obtained from 10 day old embryonic chicken tissue.
This was unexpected!
This was unexpected!
Cells: The cells were confirmed to be of chicken origin based on PCR to the mitochondrial genome. The cells are not engineered or genetically modified. The cell line was spontaneously immortalized and considered genetically stable (12-14% of cells measured were polyploid).
Cells: The cells fail on two measures of tumorigenicity. They fail to grow in soft agar & fail to grow into tumors when injected into live chickens (these experiments were performed by the original cell line developers).
Thus, you're not "eating cancer."
Thus, you're not "eating cancer."
https://twitter.com/elliotswartz/status/1625559396704215040?s=20
Process: Cells are cultured in 1000L bioreactors. No scaffolds are used to make the product. No differentiation step.
GOOD Meat currently uses a contract manufacturer JOINN Biologics (joinnbio.com) to make their product.
This was also somewhat of a surprise.
GOOD Meat currently uses a contract manufacturer JOINN Biologics (joinnbio.com) to make their product.
This was also somewhat of a surprise.

Media: Cells are grown in a media that does not contain any antibiotics.
This is consistent w/ our expectations for the industry. Decoupling meat production from antibiotics is a huge benefit of #cultivatedmeat.
gfi.org/blog/cultivati…
This is consistent w/ our expectations for the industry. Decoupling meat production from antibiotics is a huge benefit of #cultivatedmeat.
gfi.org/blog/cultivati…
Media: The media contains FBS. Using serum albumin as a proxy, only a trace amount of serum is left after washing the product (in a salt solution).
Bovine serum albumin is present at concentrations ~12x lower than that found in milk. This was thus deemed safe for consumption.
Bovine serum albumin is present at concentrations ~12x lower than that found in milk. This was thus deemed safe for consumption.
Media: But I've been told companies have found alternatives to serum!
They have. @GOODMeat filed an amendment, which was approved, for serum-free media production in Singapore earlier this year. A similar process will take place in the United States.
techcrunch.com/2023/01/18/goo…
They have. @GOODMeat filed an amendment, which was approved, for serum-free media production in Singapore earlier this year. A similar process will take place in the United States.
techcrunch.com/2023/01/18/goo…
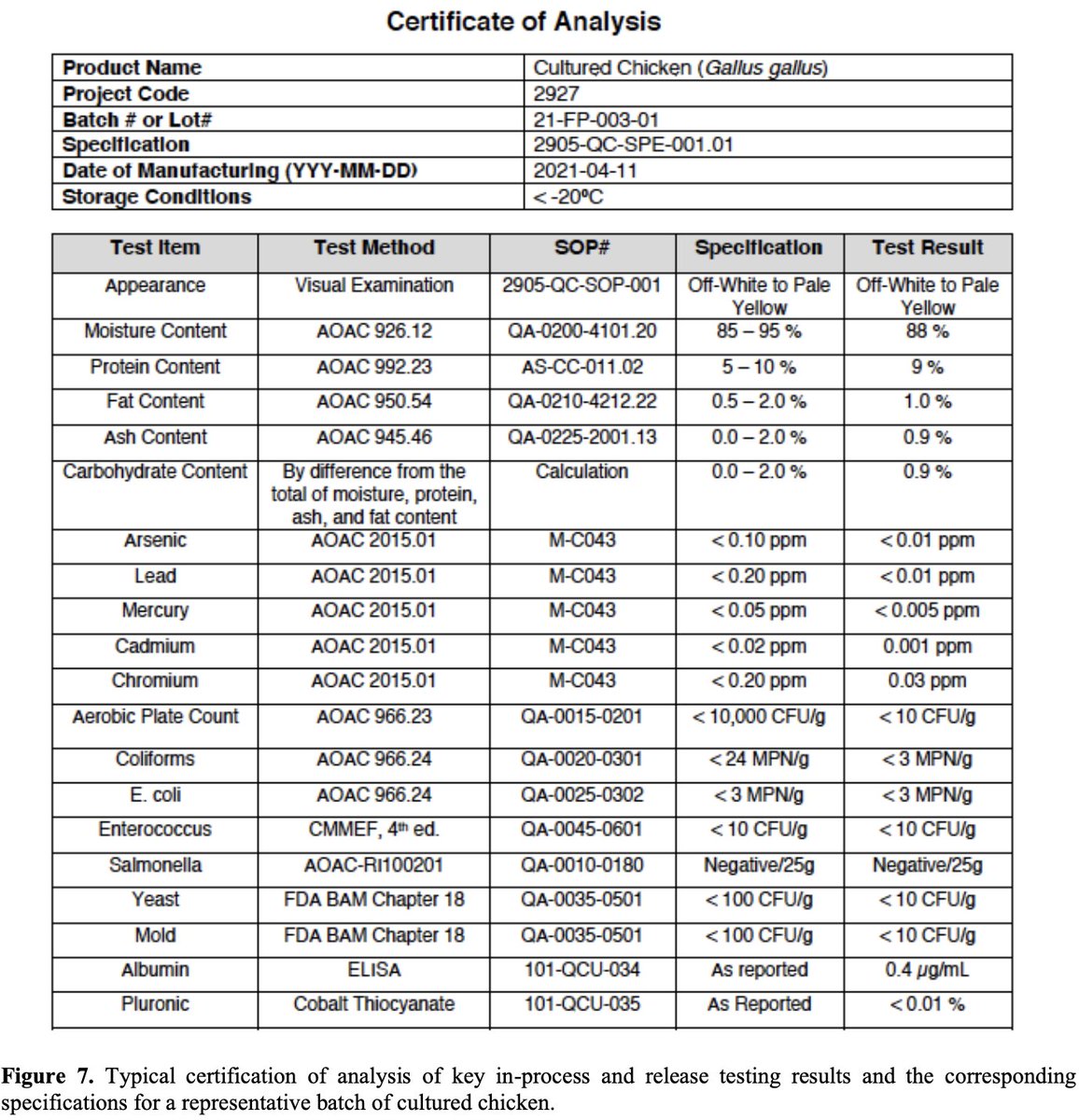
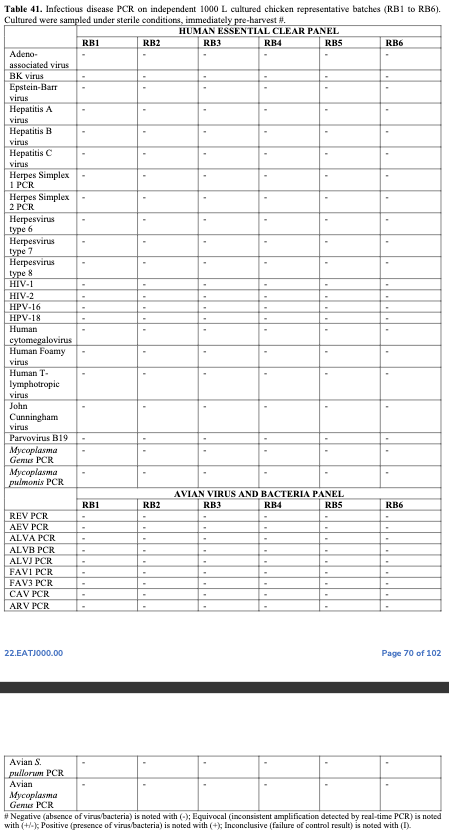
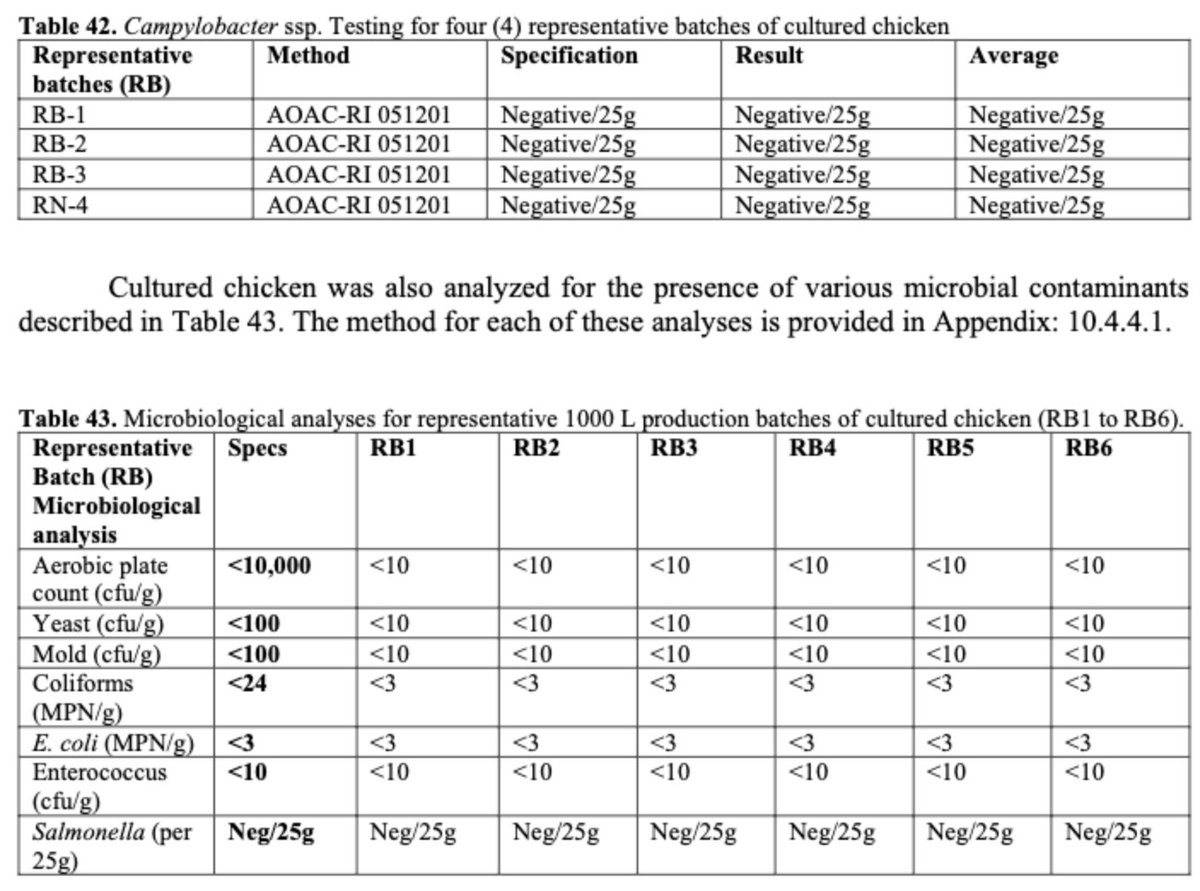
Safety: Batches of end product were tested for a variety of pathogens, including human and avian viruses & common foodborne contaminants such as E. coli and Salmonella. All negative.
Note how often chicken at your local grocery store tests positive for these contaminants.


Note how often chicken at your local grocery store tests positive for these contaminants.

Product: The cultivated chicken cells are intended to be used as an ingredient in a variety of products at 60-75% (w/w).
Shown here are proximates & heavy metals. Heavy metals are notably much lower than safe specifications.
Shown here are proximates & heavy metals. Heavy metals are notably much lower than safe specifications.
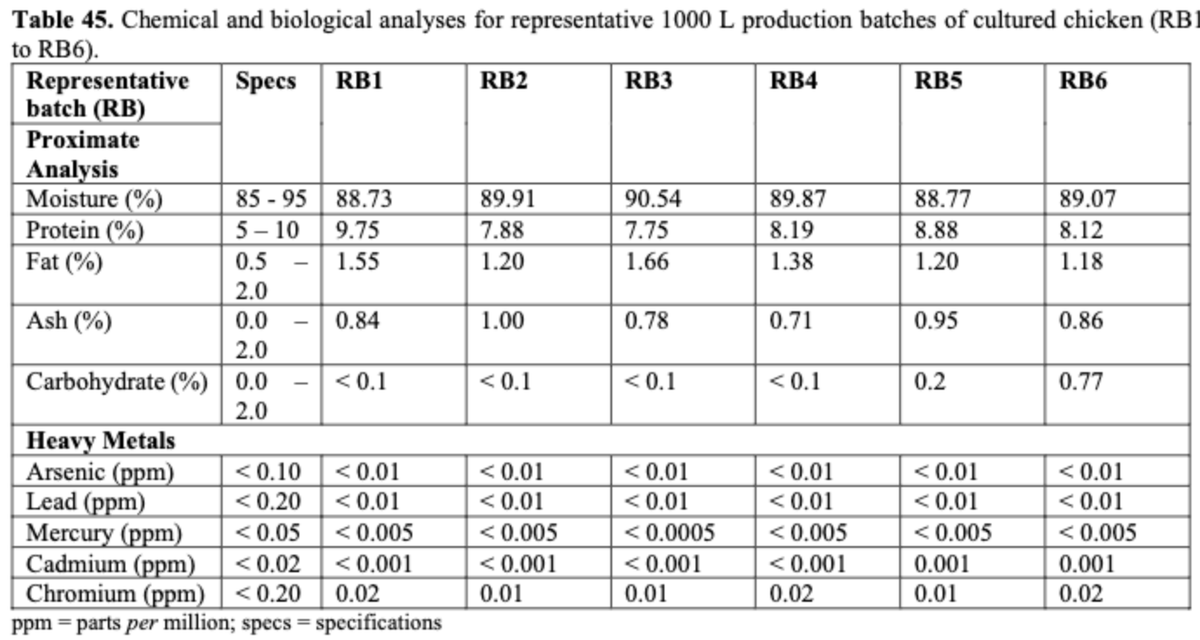
Nutrition: Overall protein and fat content when normalized on a dry weight basis is similar to conventional chicken breast.
High ash content is due to residual salts from salt wash steps.
High ash content is due to residual salts from salt wash steps.
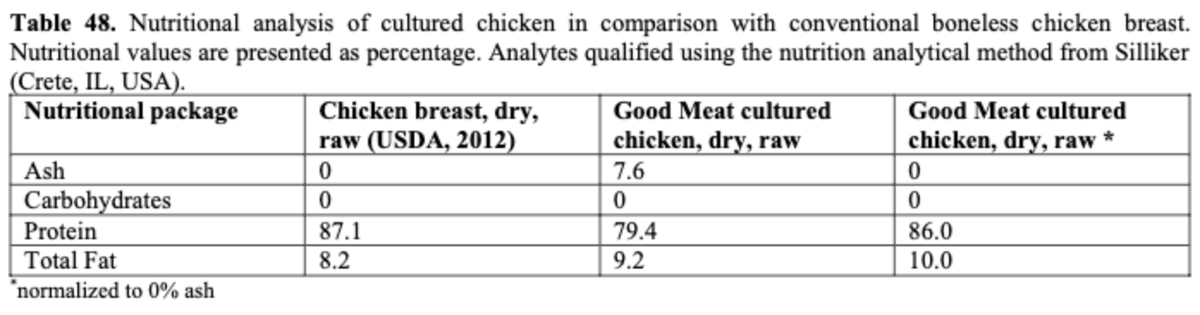
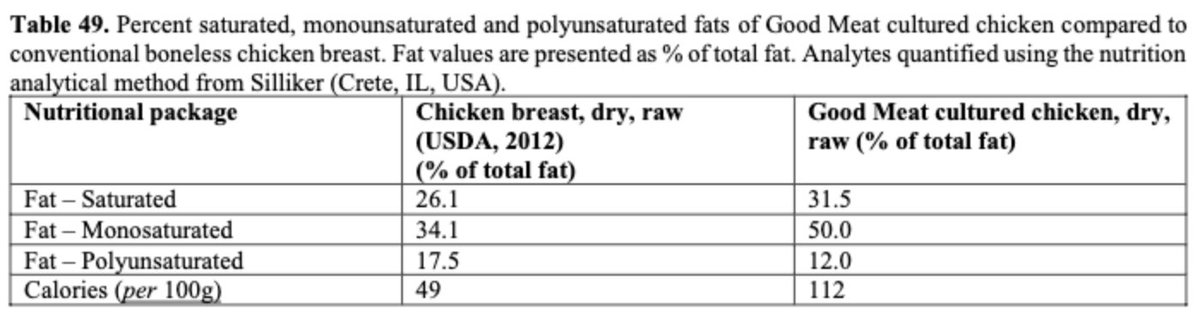
Nutrition: Amino acid & fats data.
The firm states overall amino acid content is reduced but the proportion is ~same. (Is this because the cells are not actual skeletal muscle)? FDA says fat content is broadly similar.
Cholesterol (122 mg/100g) is ~same as conventional chicken.

The firm states overall amino acid content is reduced but the proportion is ~same. (Is this because the cells are not actual skeletal muscle)? FDA says fat content is broadly similar.
Cholesterol (122 mg/100g) is ~same as conventional chicken.

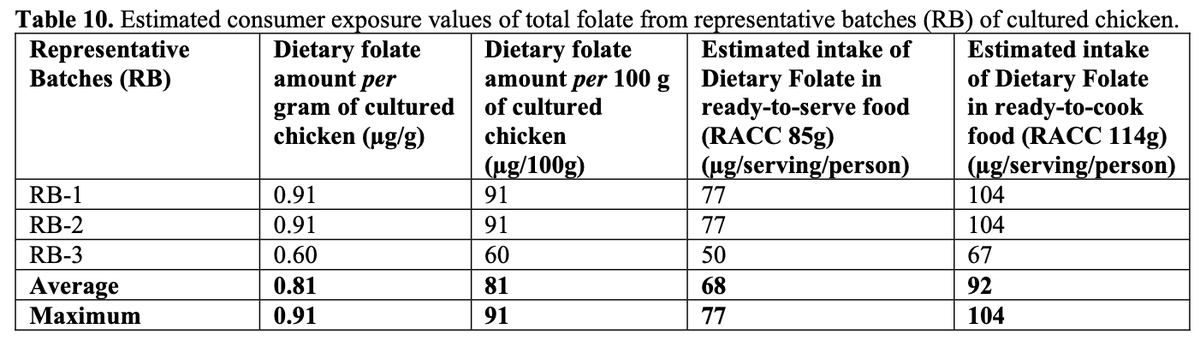
Nutrition: Full vitamin and mineral content is not provided. But some were measured to establish safety of their upstream inputs in the media. Those included iron and folate levels. 


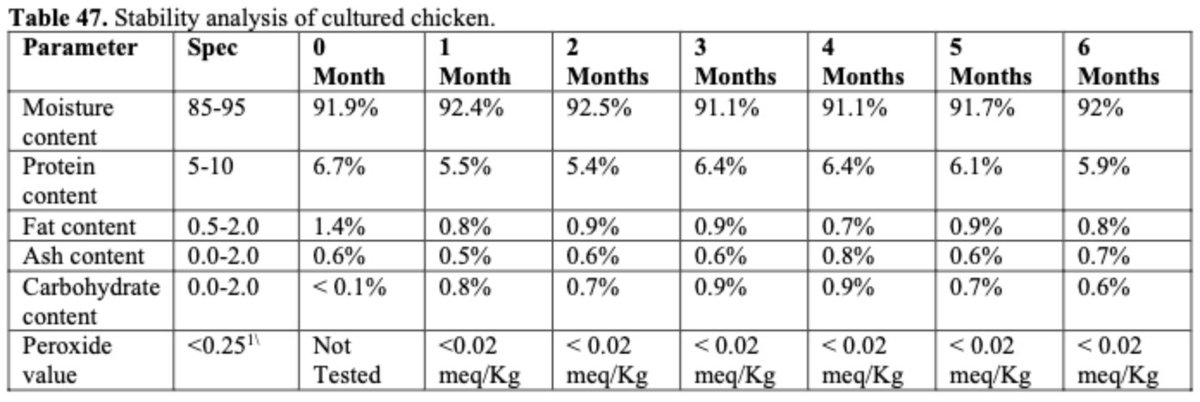
Stability: They tested stability based on composition and oxidative state w/ product frozen over 6 months. No significant changes were noted. 
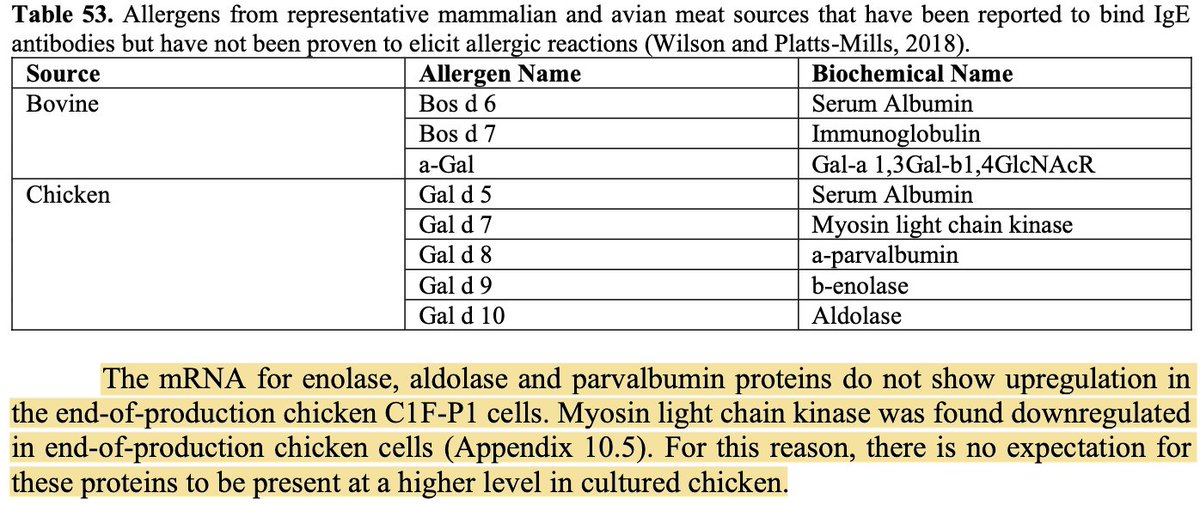
Allergens: No common allergens, including those found in chicken eggs, were expressed in the final product. As noted previously, low levels of bovine serum albumin are present due to the use of serum in the production process (for now). 
What are the next steps?
Before being sold, GOOD Meat must obtain a USDA grant of inspection, where the facility will be overseen similarly to other meat & poultry facilities. USDA will also make labeling decisions.
See here for full details:
gfi.org/resource/fda-c…
Before being sold, GOOD Meat must obtain a USDA grant of inspection, where the facility will be overseen similarly to other meat & poultry facilities. USDA will also make labeling decisions.
See here for full details:
gfi.org/resource/fda-c…
See this thread for an overview of the other completed FDA pre-market consultation to date (also of cultivated chicken).
https://twitter.com/elliotswartz/status/1597231272681566208?s=20
@threadreaderapp unroll
• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter