Long thread Sunday. I’m not able to make the trip to LA for #ASGCT23, but that didn’t stop me from combing through 1,700 abstracts to bring you just as many tweets on the highlights. Some key themes and tidbits once again (1/26)
Before we dig in, a refresher on the themes I called out last year, as many are still relevant and highly represented this year. I’ll try to focus on new areas (2/26)
https://twitter.com/prof_oak_/status/1522587775014428673?s=46&t=mkJGGldDdQyuED4alkfLhw
#1: Delivery (1/2) - to no one’s surprise, delivery remains a core theme. I flagged the saturation of novel AAV capsid abstracts last year, and 2023 is no different, but it also includes a broader and more diverse set of technologies than ever before (3/26)
#1: Delivery (2/2) - Big year for RNPs [29, 228, 319, 1687], Ultrasound / Electroporation [25, 31, 875, 1301, 1553], mAb-Conjugated AAVs [ $REGN - 42, 468, $RGNX - 447] Kidney Tropism [331, 450, 1546, 1684], and of course a plethora of abstracts on extra-hepatic LNPs (4/26)
#2: Editing Toolbox (1/2) - if you follow the literature closely, you know that there has been an Cambrian Explosion of new gene editing modalities in the past few years, many of which will be on display (5/26)
#2: Editing Toolbox (2/2) - beyond the stalwarts, some intg. presentations on CASTs (338, 1429), Recombinases (520), FiCAT (814), Epigenetic Editing (155), BE for Transversions (257), non-Cas9 variants of BE / PE (256, 1346), dCas9-SSAP fusions (261), and LT-CRISPR (1387) (6/26)
#3: Pharma Presence (1/3) - encouraging to see an increasingly large presence from pharma at ASGCT. By my count, there are >20 presentations from ~7 global pharmas - no doubt that select players have made investing in CGTx a priority. Some of the standouts: (7/26)
#3: Pharma Presence (2/3) - (1) $REGN mAb-conj. AAV platform for muscle (CACNG1) & CNS (TfR1) tropism; (2) color on several late-stage efforts from $SNY, incl. NHP data for AAV programs - vectorized DMPK KD [1042], MLD [1071] - as well as a $GBIO F8 ceDNA comp [966] (8/26)
#3: Pharma Presence (3/3) - (3) $AZN investing in gene editing - presenting on a novel Type II nuclease [526], small molecules to enhance HDR efficiency [334], and replication-competent AAV episomes (cool!) [1360] (9/26)
#4: In Utero Medicine (1/2) - continuing its momentum with several presentations spanning neurodevelopmental, pulmonary, and epidermal disorders, including NHP data in some cases [38, 438, 842, 873, 1382] (10/26)
#4: In Utero Medicine (2/2) - I’ve tweeted extensively on this, so I won’t rehash it here, but in utero intervention could be transformational for many rapidly progressing diseases - much to be de-risked, but the long-term potential for patients is significant (11/26)
Moving beyond themes over to notable individual presentations, corporate or otherwise: (12/26)
A few intg. ophtho datasets. Success for $APLS & $ISEE naturally breeds ‘one-and-done’ competition - $RGNX [364] and $ADVM [976] presenting in-house anti-C5 and CFI AAV constructs, respectively. Anyone think GTx for GA will go any differently than it has for wet AMD? (13/26)
Meanwhile, $BEAM presenting initial AAV BE data in NHPs for Stargardt [308], a 1st dataset of its kind. Achieve editing efficiencies of ~50% in cones, ~25% in rods, ~70% in RPE cells, but unclear how these are calculated (within the bleb, extrapolation of bleb, etc.) (14/26)
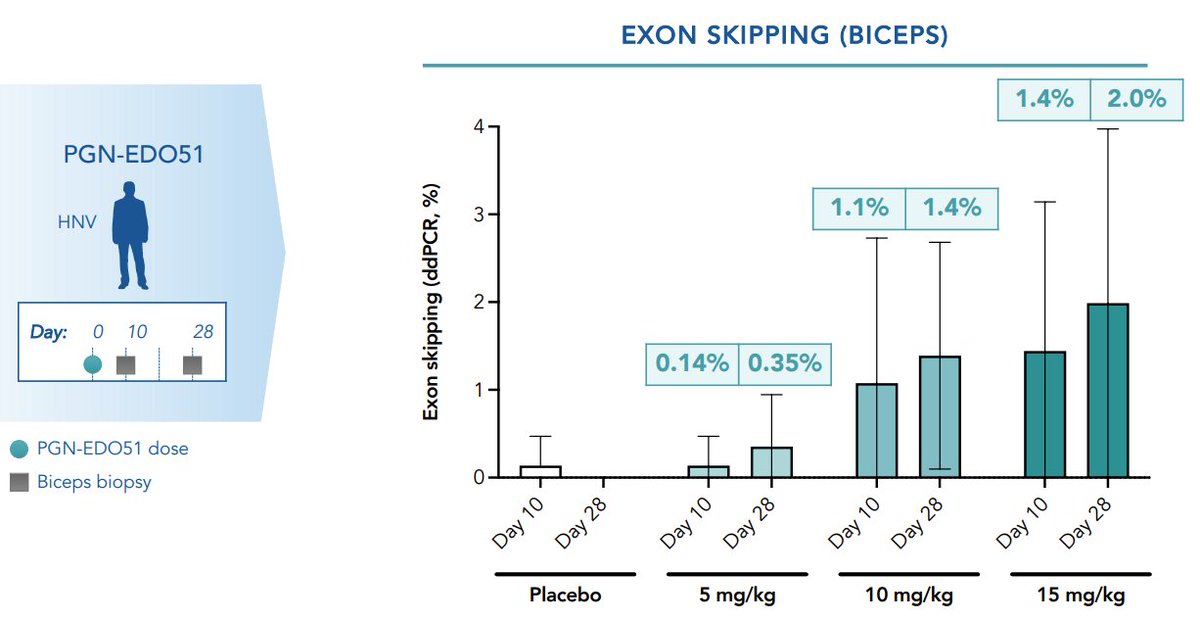
In DMD, an encouraging case report from Columbus Children’s vectorized exon skipping program [802]. Latest patient, dosed much earlier (in infancy), showed significantly better results (recall, the first two patients showed very low levels of skipping / dystrophin)... (15/26)
...This patient, OTOH, showed 99% exon skipping, 95% dystrophin+ fibers, and 88% dystrophin vs. WT on biopsy. Really remarkable, and probably supports the case for AAV treatment as early as possible in DMD, irrespective of mechanism (16/26)
Also in AAV land, 1st pres on an IgM cleaving enzyme to enable AAV re-dosing [210]. Work on IgG degraders previously received a lot attention; Asokan lab show the disc. of an IgM degrader & feasibility of combining the two for more robust amelioration of anti-AAV immunity (17/26)
Similarly, 2 pres from $SNY & $RHHBY evaluating kinase inhibitors, IRAK4 [725] & Src [1693], resp., for prevention of AAV immunity, with promising results. A lot going on in this space - combining new learnings usher a future in which AAV doesn’t have to be ‘one-and-done’ (18/26)
On the Cell Therapy side, a presentation from Novartis showing KO of endogenous TCR is deleterious to CAR-T function [269]; corroborates some academic work that was released previously - potentially why $NVS has never invested in allogeneic? (19/26)
Meanwhile, a new approach to immune evasion for allogeneic cell therapies from Michel Sadelain [221]; leveraging the biology of viral proteins from HIV, CMV, EBV, and KSV for immune evasion... (20/26)
...all showed various levels of MHC Class 1 downregulation, but NEF from HIV seems to have emerged as the winner via a pleiotropic mechanism spanning MHC downregulation AND attenuation of T cell function. Wonder what the theoretical safety risks of this might be (21/26)
Moving to HSCs, $GRPH is presenting on “Molecular Dynamics of Genome Editing with CRISPR/Cas9 and rAAV6 in Human HSPCs” [515] - potentially some incremental data on the drivers of pancytopenia? (22/26)
Similarly, an interesting study from SR Tiget looking at cellular toxicity of BE and PE in HSCs [98]; BE induced p53, had some LT engraftment disadvantages, and had some DSBs / large deletions / insertions (less than Cas9 in all cases)... (23/26)
...PE similarly had unintended outcomes and induced p53 (less than Cas9; comparison to BE not quantified), as well as a few unique transcripts (likely related to RT sensing), but did not show any detrimental impact on engraftment in serial transplant experiments (24/26)
Finally, much progress in B Cells. Be Bio seems to have robustly solved editing (60% HDR efficiency [119]). Also eye catching was [575], showing xenograft efficacy of B cells eng. to secrete CD19 & CD33 TCEs - translational relevance debatable; cool factor indisputable (25/26)
Okay, that’s all from me. Wish I could make it in person, but looking forward to watching from afar, nonetheless. Let me know what I missed! (26/26)
https://twitter.com/Prof_Oak_/status/1655175050943668226?s=20
• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter