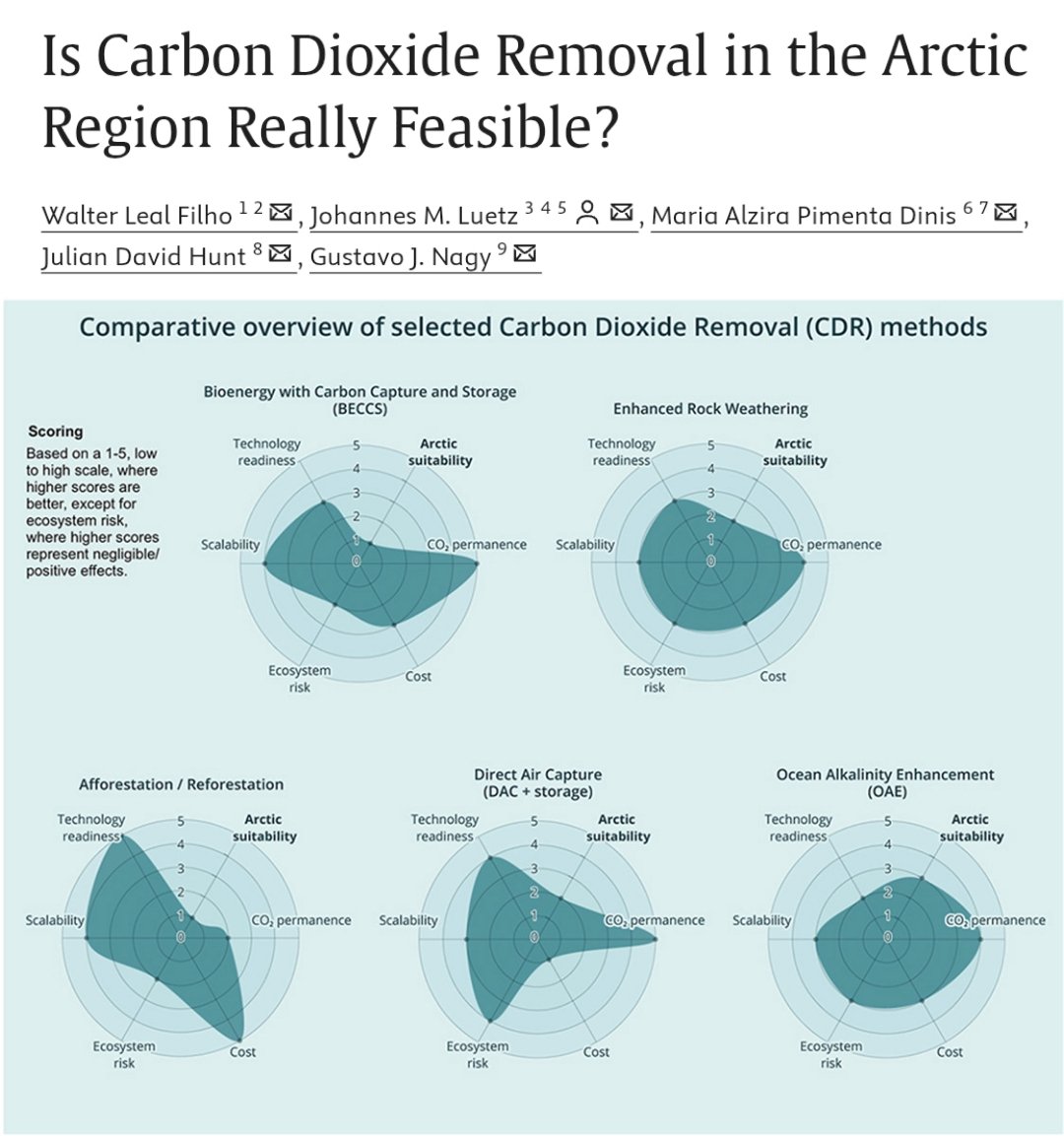
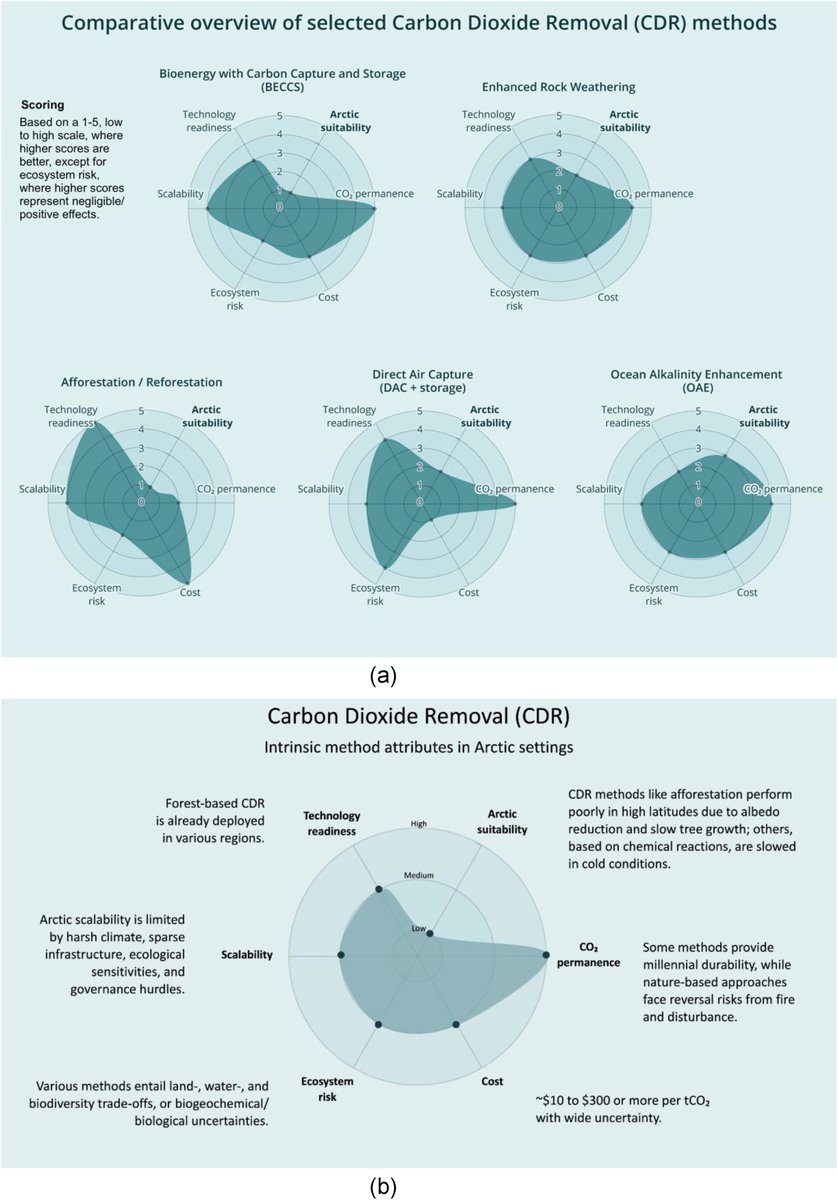
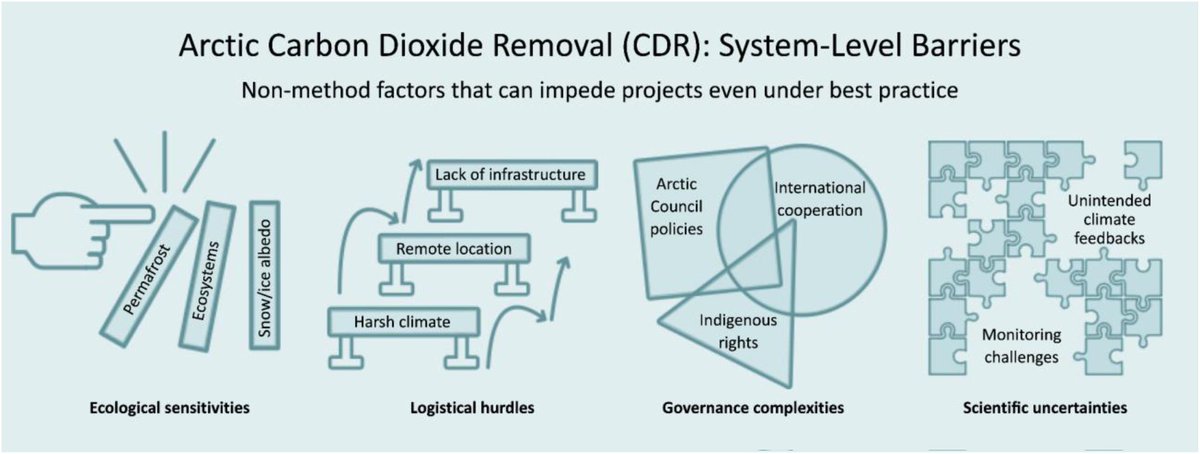
🚨NEW PAPER🚨
"Among #sequestration methods, CO2 #injection into oceans is of great primacy due to the oceans’ large sequestration ability. However, there are concerns about the changes in H2O pH as CO2 is injected into oceans."
On this point new study is conducted, details🧵⬇️

"Among #sequestration methods, CO2 #injection into oceans is of great primacy due to the oceans’ large sequestration ability. However, there are concerns about the changes in H2O pH as CO2 is injected into oceans."
On this point new study is conducted, details🧵⬇️

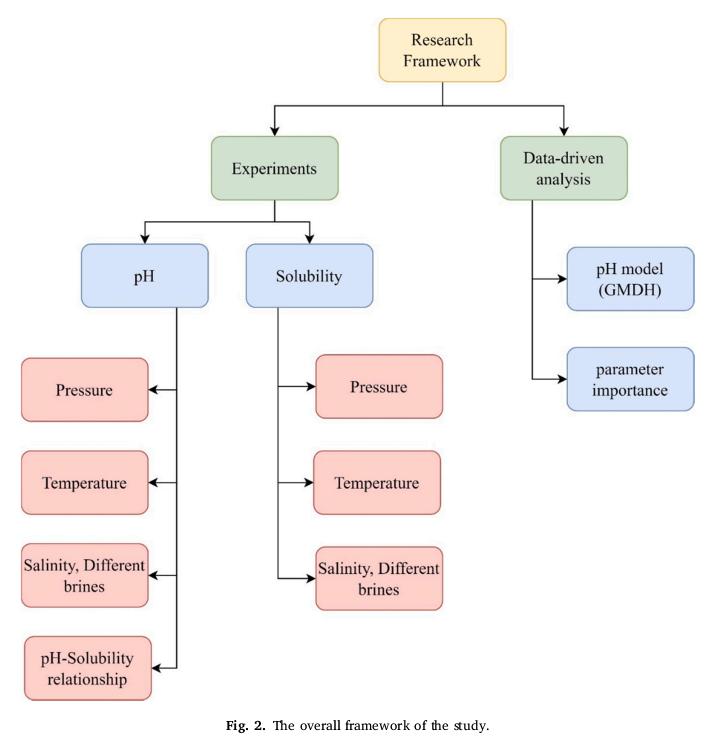
🌊 Researchers "experimentally measure the pH and solubility at #pressures up to 400 atm, #temperatures between 283 and 298 K, and different aqueous solutions in a high-pressure #autoclave reactor."
2/10
2/10

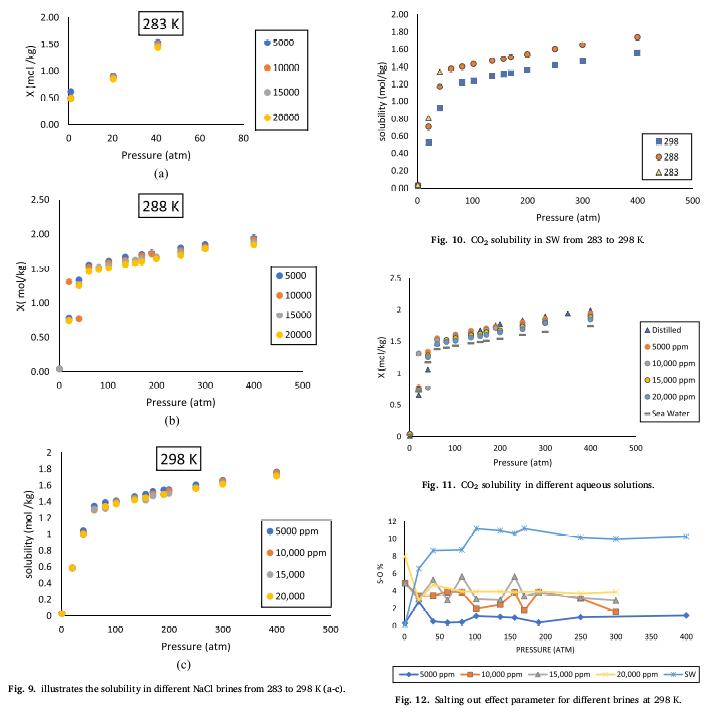
🌊The results of this research "indicated that increasing pressure increases the #solubility of CO2 in aqueous solutions, resulting in lower pH values. In contrast, increasing #salinity and #temperature lowers the solubility and, as a result, increases the system's pH."
3/10
3/10

🌊"Among all the tested aqueous solutions, the synthetic seawater mimicked that of a potential #injection point in the #SouthChina sea, exhibiting the highest salting-out effect and, therefore, the lowest solubility (i.e., the highest pH)."
4/10
4/10
"The experimental dataset of this study was fed to a machine learning algorithm, Group modeling data handling(GMDH), to develop an explainable solubility model. The model could predict the pH as a function of solubility, temp, pressure, & salinity with an accuracy of 0.87."
5/10

5/10

"The #pH values from the model were compared by the researchers of this study to those from previous studies, and a good agreement among the values was found."
6/10
6/10
Lastly, "a parameter importance analysis was conducted to shed further light on the model's performance. #Pressure and #temperature were found to be the most and the least influential factors, respectively."
7/10

7/10


"As the implantation of the technology is currently being considered in China, the current study can pave the way to better understand the interactions & mechanisms involved in conditions representative of ocean #sequestration before large-scale operations, study concluded."
8/10

8/10


Read the study entitled: "Probing Solubility and pH of CO2 in aqueous solutions: Implications for CO2 injection into oceans" here ⬇️
sciencedirect.com/science/articl…
#OceanSequestration 🌊
#CarbonDioxideInjection
9/10
sciencedirect.com/science/articl…
#OceanSequestration 🌊
#CarbonDioxideInjection
9/10
• • •
Missing some Tweet in this thread? You can try to
force a refresh