CAPE COD trial:
31 ICUs in France
Required MV, HFNC, or NRB
Randomized to Hydrocort 200mg x 4 days or placebo
At day 4, if still sick based on pre-specified criteria, re-randomized to receive steroids until day 8 or 14
31 ICUs in France
Required MV, HFNC, or NRB
Randomized to Hydrocort 200mg x 4 days or placebo
At day 4, if still sick based on pre-specified criteria, re-randomized to receive steroids until day 8 or 14
Primary outcome:
Steroids: 6.2% mortality by day 28
Placebo: 11.9% mortality by day 28
Persisted through day 90
Steroids: 6.2% mortality by day 28
Placebo: 11.9% mortality by day 28
Persisted through day 90
For prespecified subgroup analysis of patients not intubated at baseline, HC group was less likely to get intubated than placebo group 
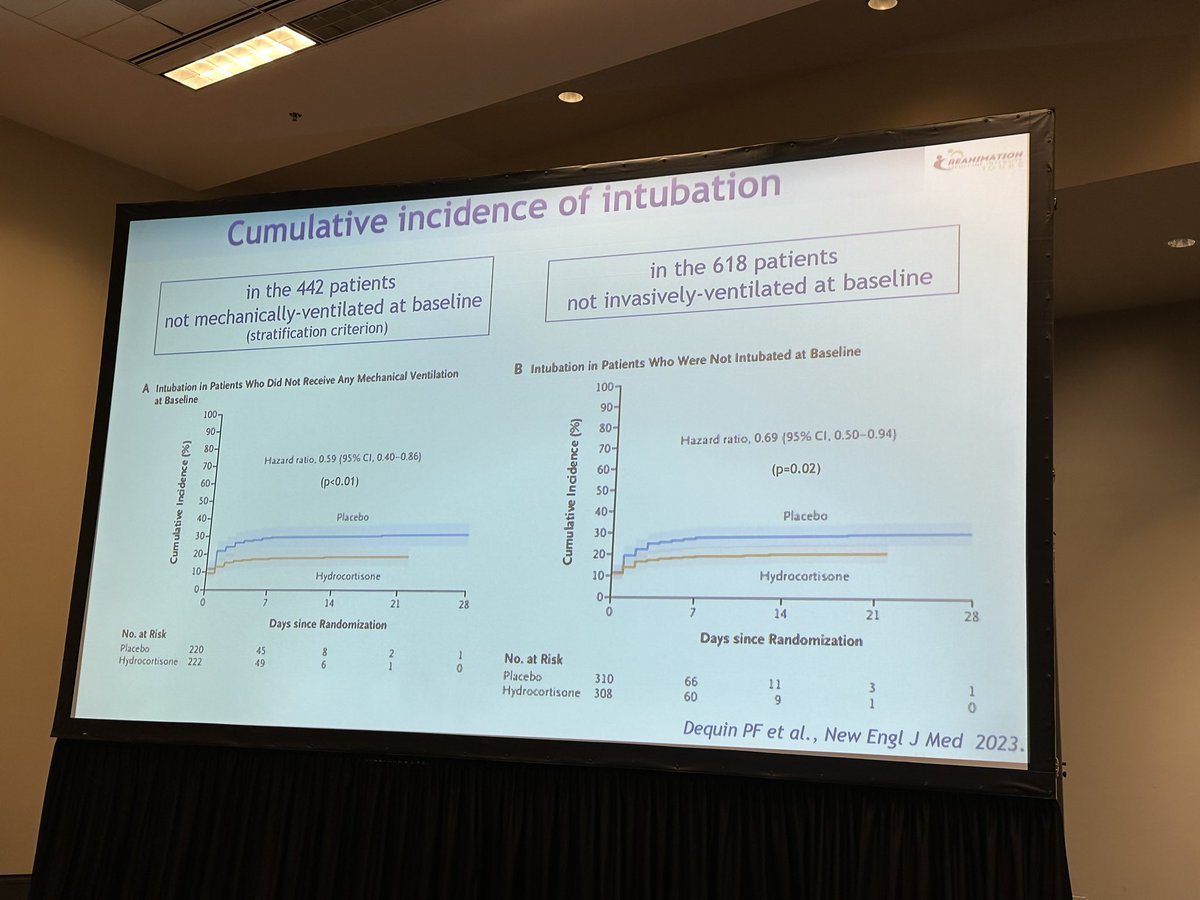
Safety Outcomes:
No difference in hospital acquired infections.
Increase incidence of hyperglycemia and insulin use in HC group
No difference in hospital acquired infections.
Increase incidence of hyperglycemia and insulin use in HC group
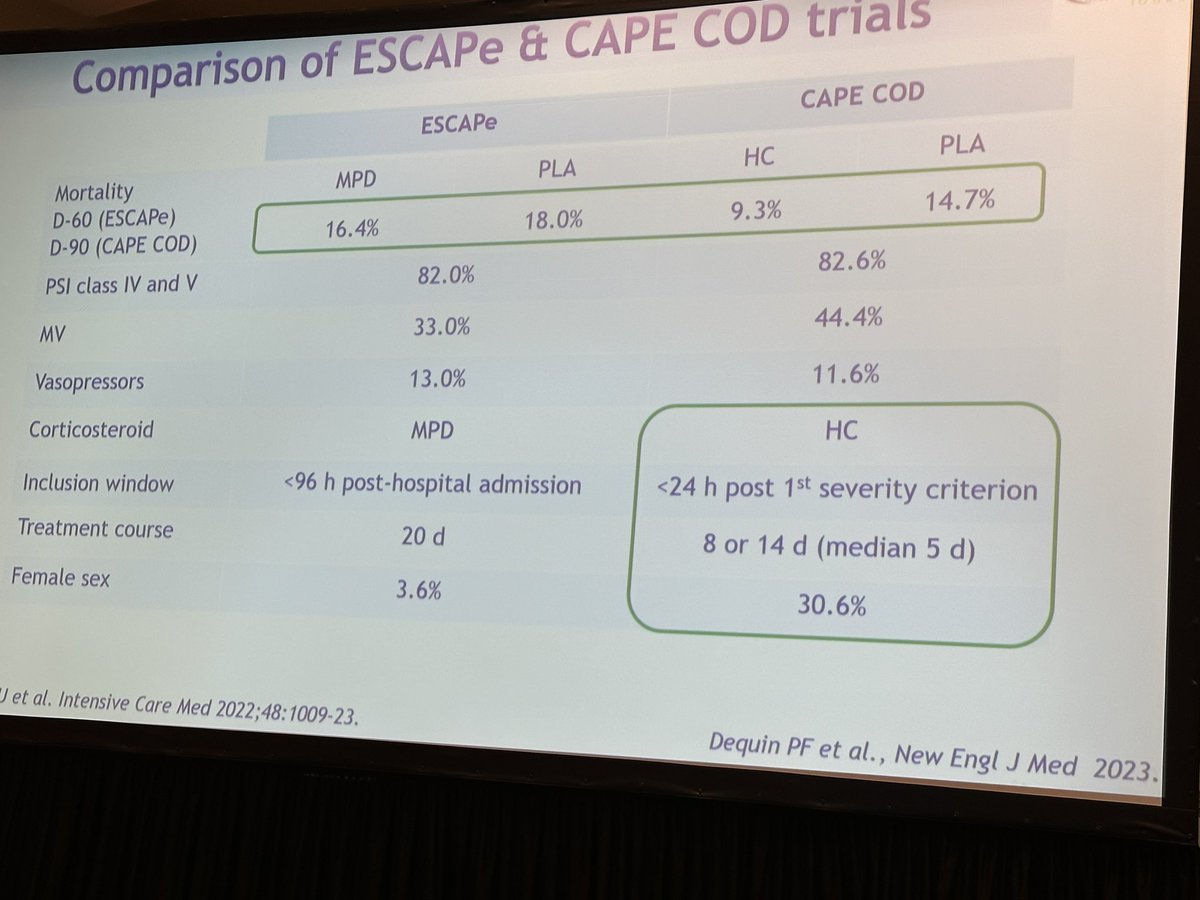
Trying to contextualize given the ESCAPE trial (methylpred for CAP) and CAPECOD. ESCAPE trial showed no difference.
- Diff steroid use (HC VS MPD)
- Earlier enrollment in CAPECOD
- Shorter tx duration in CAPECOD
- 30% female Vs 3% female
- could be related to HTE
- Diff steroid use (HC VS MPD)
- Earlier enrollment in CAPECOD
- Shorter tx duration in CAPECOD
- 30% female Vs 3% female
- could be related to HTE
Final conclusions:
Low dose, early, short course IV Hydrocortisone increases survival to day 28 and likely decreases rates of intubation and vasopressor use.
Low dose, early, short course IV Hydrocortisone increases survival to day 28 and likely decreases rates of intubation and vasopressor use.
NEJM Editor:
Finally a critical care trial that reduces mortality!
This trial may have the potential to change clinical practice guidelines
Need more evaluation of HTE
Only 6% pts immunocompromised so caution in this population
Finally a critical care trial that reduces mortality!
This trial may have the potential to change clinical practice guidelines
Need more evaluation of HTE
Only 6% pts immunocompromised so caution in this population
Question:
1. Any evidence of HTE based on blood work, eg CRP
A - analysis not done yet! Blood work is collected
2. Effect on LTO?
A: LTO process ongoing but relatively limited
1. Any evidence of HTE based on blood work, eg CRP
A - analysis not done yet! Blood work is collected
2. Effect on LTO?
A: LTO process ongoing but relatively limited
• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter



















