I have spoken about the importance of minimally clinically important differences (#MCID)
before in relation to sample sizes but how do you decide what it should be? There was an interesting paper published this week adding to this literature 1/8
#MethodologyMonday
before in relation to sample sizes but how do you decide what it should be? There was an interesting paper published this week adding to this literature 1/8
#MethodologyMonday
The MCID drives the sample size - it is the minimum clinically important difference you set your trial to detect. Set the MCID too small and the sample size will be much larger than needed; but make the MCID too big & your trial will miss clinically important effects 2/8
There are different methods of calculating #MCIDs - the DELTA project is a great resource in this regard. 3/8
DELTA: journalslibrary.nihr.ac.uk/hta/hta18280/#…
DELTA: journalslibrary.nihr.ac.uk/hta/hta18280/#…

DELTA identified seven main methods for calculating MCIDs - anchor, distribution, health economic, opinion-seeking, pilot study, review of evidence base and standardised effect sizes. 4/8 

The DELTA team also published a guide on how to choose an appropriate MCID (in the DELTA-2 project) 5/8
bmj.com/content/363/bm…
bmj.com/content/363/bm…

This week, Wang et al also published a useful framework to help guide which MCID to use (this one focussing on patient reported outcomes) especially if there are a selection of different possible MCID estimates available. 6/8
bmj.com/content/381/bm…
bmj.com/content/381/bm…

The framework proposes a systematic step-by-step action plan to ensure the choice of MCID is both methodologically sound and contextually relevant. They provide a detailed flow chart to navigate the process 7/8 
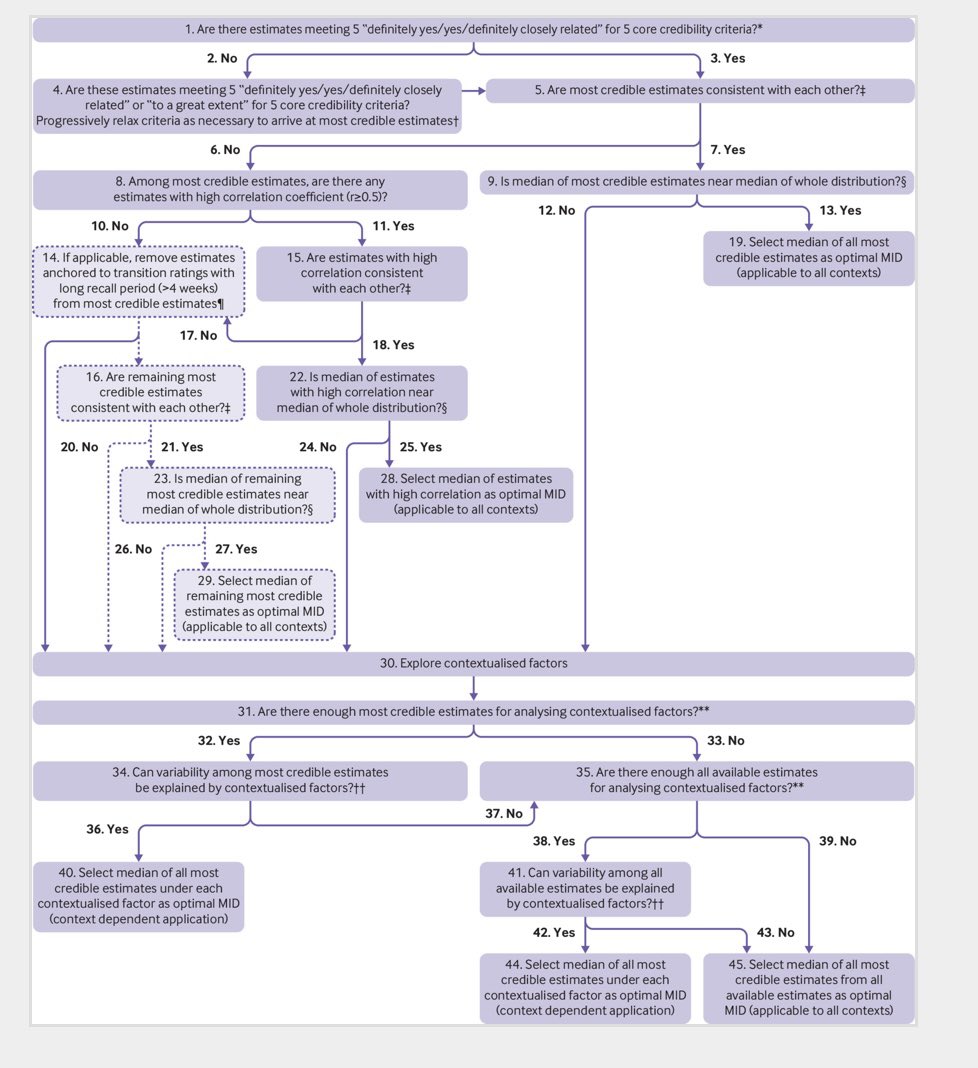
Repeated studies have found that the choice of MCID is often not defined or justified in trial protocols or reports. As with most other aspects of trials, reporting needs to be better 8/8
• • •
Missing some Tweet in this thread? You can try to
force a refresh





