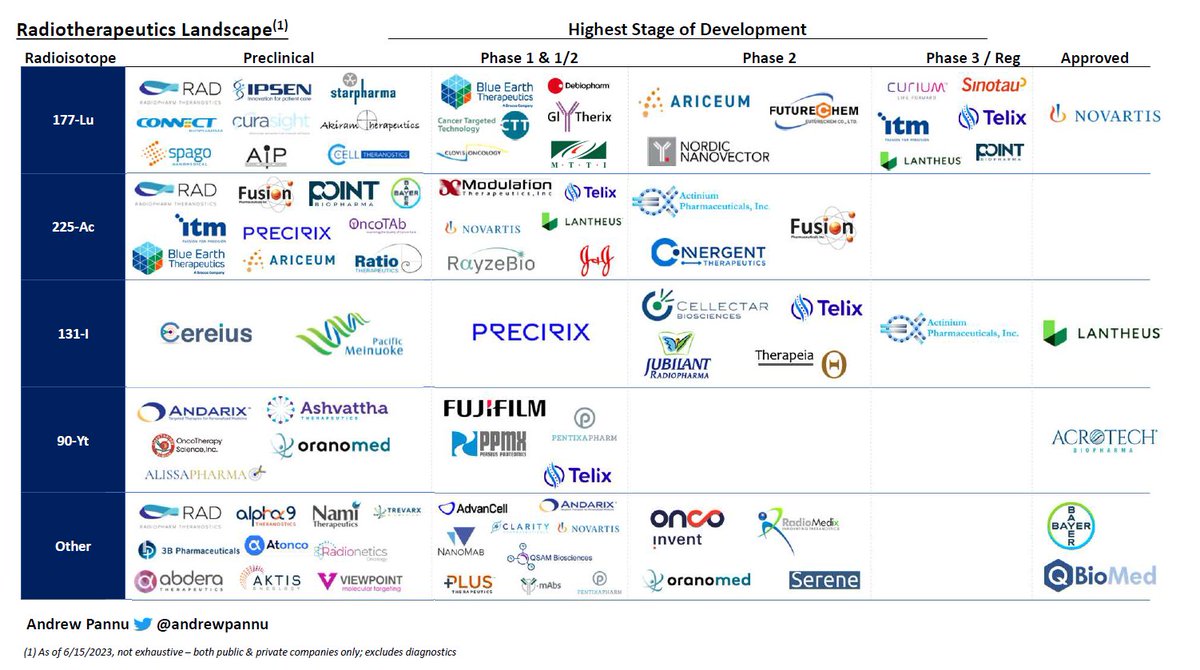
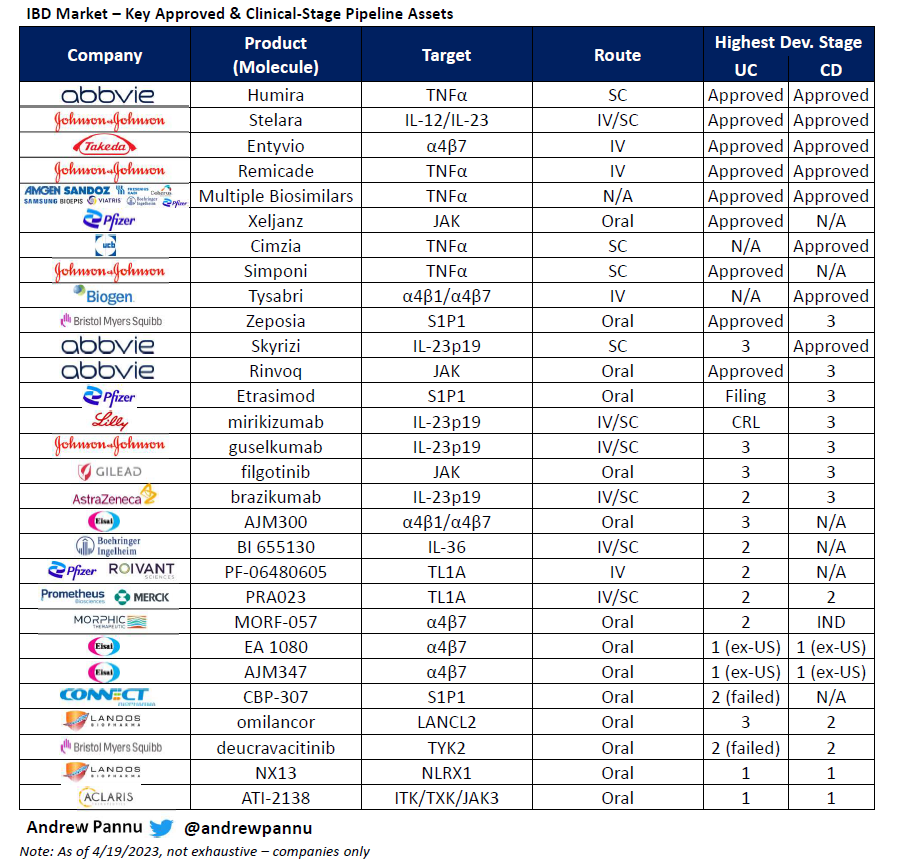
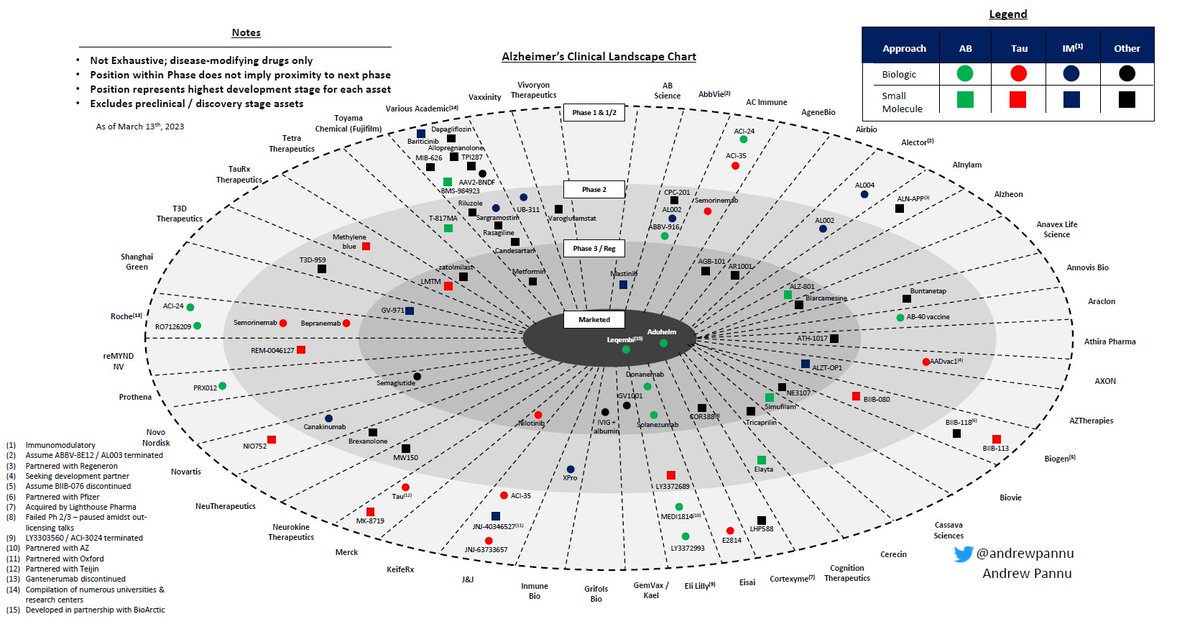
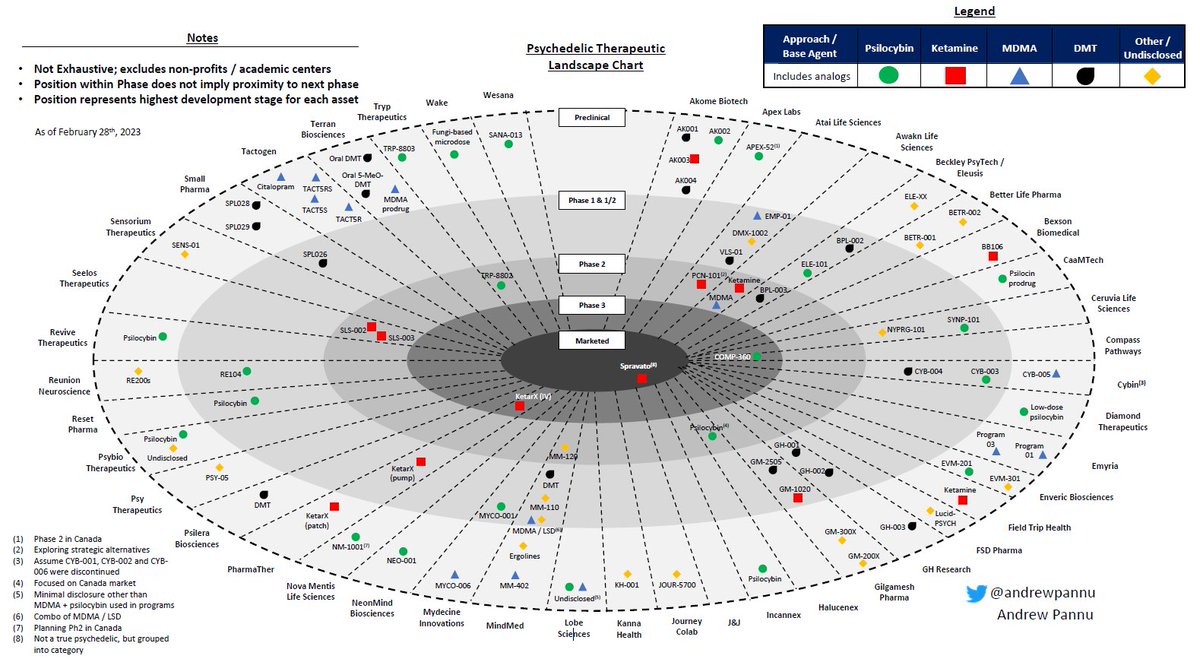
Gene editing landscape
I pulled together 42 public & private companies and charted the preclinical & clinical assets of each, segmented by approach & editor
Some takeaways:
I pulled together 42 public & private companies and charted the preclinical & clinical assets of each, segmented by approach & editor
Some takeaways:

In the context of therapeutics, gene editing is the insertion, deletion or replacement of sections of a patient's DNA to treat disease
The Cowen team pulled together the helpful graphic below noting the nuances between gene therapy and gene editing
The Cowen team pulled together the helpful graphic below noting the nuances between gene therapy and gene editing

While the above analysis is not exhaustive (there are >250 active gene editing programs), it does help illustrate how early the space is
~85% of all programs are preclinical, with everything else in Ph1/2 except $CRSP & $VRTX exa-cel (submitted BLA, PDUFAs in Dec'23 & Mar'24)
~85% of all programs are preclinical, with everything else in Ph1/2 except $CRSP & $VRTX exa-cel (submitted BLA, PDUFAs in Dec'23 & Mar'24)
Things to watch as the space matures:
(1) Patient & indication selection: 1st gen assets primarily targeted severe patient populations of monogenic diseases (i.e. SCD, TDT) with limited treatment options
Emerging assets are targeting more competitive spaces (CV, Onc, Metabolic)
(1) Patient & indication selection: 1st gen assets primarily targeted severe patient populations of monogenic diseases (i.e. SCD, TDT) with limited treatment options
Emerging assets are targeting more competitive spaces (CV, Onc, Metabolic)
It will be interesting to track patient / physician acceptance of a potentially curative but relatively new treatment vs. more established chronic treatments
(2) Reimbursement: as with gene therapies, the one-time nature of treatment will command sky-high price tags
(2) Reimbursement: as with gene therapies, the one-time nature of treatment will command sky-high price tags
Expect payor pushback in more prevalent conditions with an established, chronic & cheaper SoC
Companies should get comfortable with value-based agreements, where reimbursement is tied to treatment milestones & staged over time
Mktd. gene therapies will help set guidelines here
Companies should get comfortable with value-based agreements, where reimbursement is tied to treatment milestones & staged over time
Mktd. gene therapies will help set guidelines here
(3) CMC: this can quickly become a bottleneck for companies during clinical development & beyond, as scaling these novel processes to pivotal / commercial grade with appropriate comparability to earlier-stage trials is difficult
(4) Safety: given we are talking about permanent genome edits, this will remain in focus for the foreseeable future
The FDA has been cautious and placed lengthy clinical holds on several programs to request more data / investigate abnormalities
Minimal off-target effect is key
The FDA has been cautious and placed lengthy clinical holds on several programs to request more data / investigate abnormalities
Minimal off-target effect is key
Since CRISPR-Cas9 was invented in 2012, gene editing companies have raised >$18B (public & private)
Approval of $CRSP / $VRTX exa-cel in 2023 would thus be a watershed moment for the space, and the culmination of a decade of fast-paced innovation & investor / pharma enthusiasm
Approval of $CRSP / $VRTX exa-cel in 2023 would thus be a watershed moment for the space, and the culmination of a decade of fast-paced innovation & investor / pharma enthusiasm
Looking ahead beyond exa-cel, there are a few other catalysts to monitor:
♦ Additional NTLA-2001 data in ATTR & initiation of global pivotal study ($NTLA)
♦ First look at clinical data for a base editor via VERVE-101 ($VERV)
♦ Clinical update from EDIT-301 TDT trial ($EDIT)
♦ Additional NTLA-2001 data in ATTR & initiation of global pivotal study ($NTLA)
♦ First look at clinical data for a base editor via VERVE-101 ($VERV)
♦ Clinical update from EDIT-301 TDT trial ($EDIT)
To see my prior breakdown of the gene therapy landscape, check out the below
https://twitter.com/andrewpannu/status/1625496860084539399?s=20
That's all - If you enjoyed this, follow me @andrewpannu for more biotech charts, musings and breakdowns
If you'd like a PDF of the market landscape, you can comment below (my site is currently down)
Also, let me below if I missed any notable companies for future iterations
If you'd like a PDF of the market landscape, you can comment below (my site is currently down)
Also, let me below if I missed any notable companies for future iterations
• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter