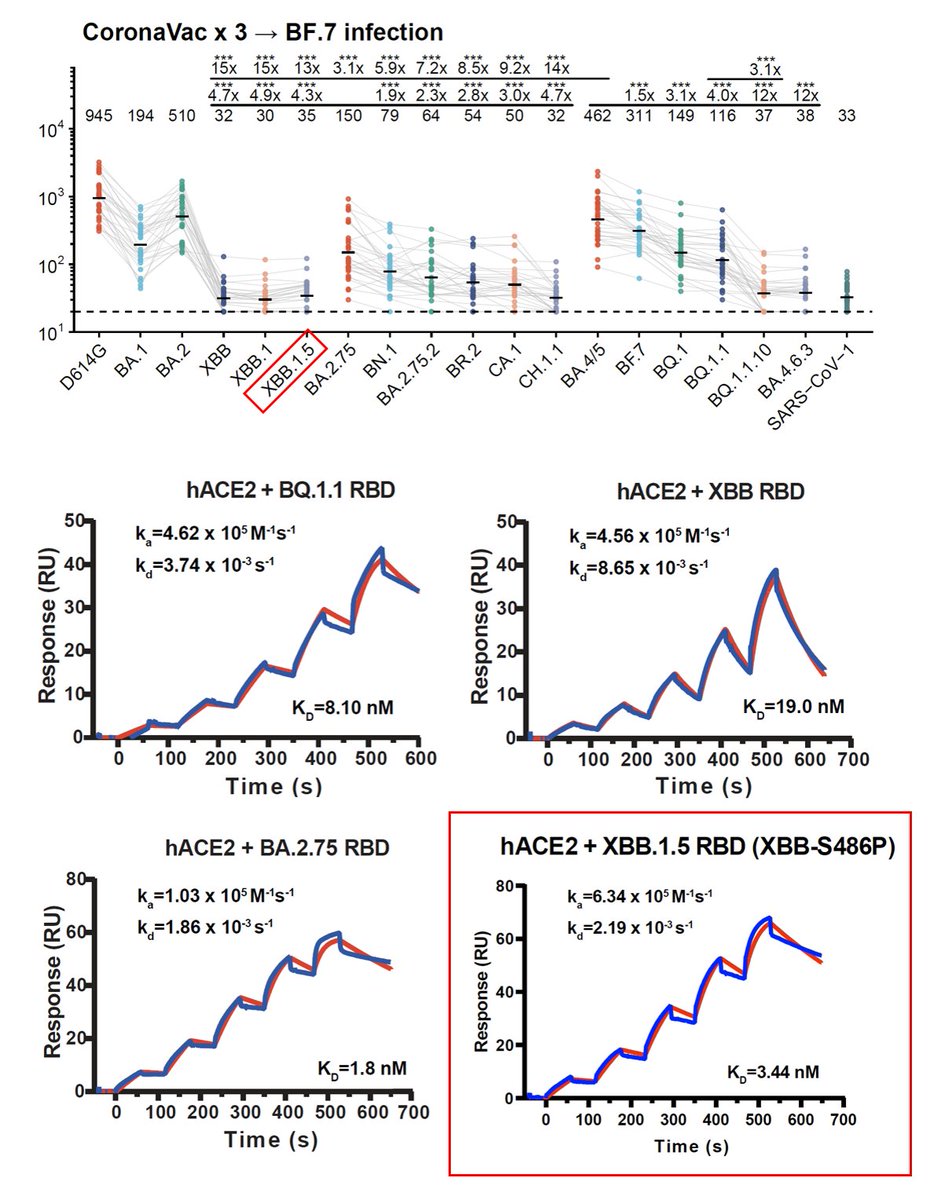
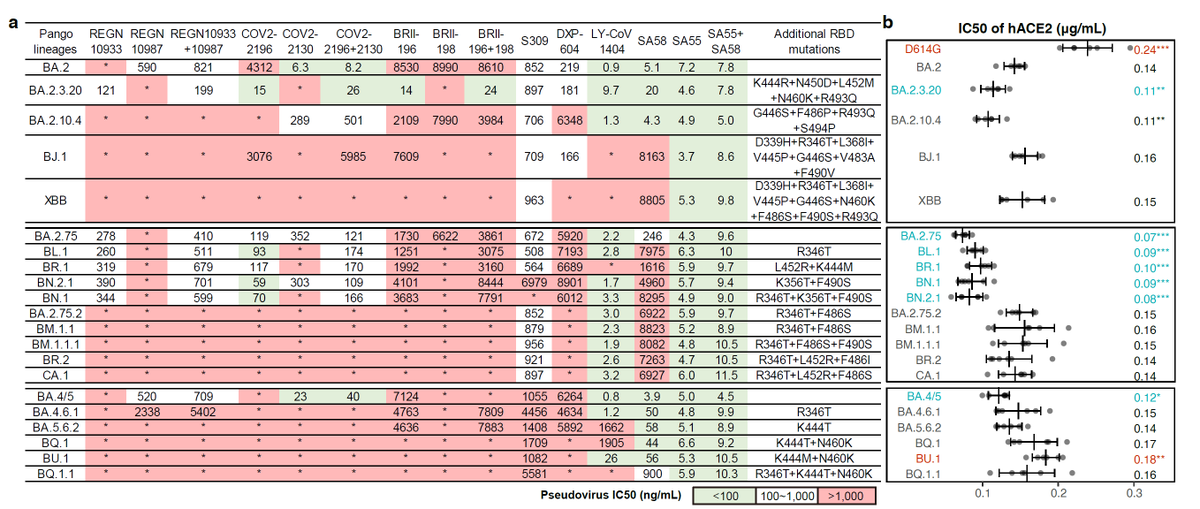
F456L-carrying XBB*, like EG.5, is rapidly rising. Meanwhile, XBB*+L455F+F456L is also growing fast. Some updates explaining their advantages:
1) F456L evades serum neutralization, even after XBB infection.
2) L455F+F456L combo adds on evasion and could also boost ACE2 binding!
1) F456L evades serum neutralization, even after XBB infection.
2) L455F+F456L combo adds on evasion and could also boost ACE2 binding!

The L455F+F456L RBD mutation combo is a very smart move by the virus (it's actually an LF->FL shift). Note that both individual L455F or F456L actually lose ACE2 binding, but together, the LF->FL shift somehow strengthened ACE2 interaction while destroying most antibody binding. 

The emergence of 455 & 456 mutations is well-predicted half-year ago by our model built on DMS. Interestingly, we recently found that F456L is much more well-tolerated on the XBB.1.5 backbone instead of BA.2, which may explain why F456L only started to rise just now. 
It can be highly expected that this winter, we will be facing XBB offspring that carry mutation combos like L455F+F456L+K478R, or even additional evasive mutations since the high ACE2 affinity could give a large buffering room for strong antibody-evading mutations to appear.
• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter