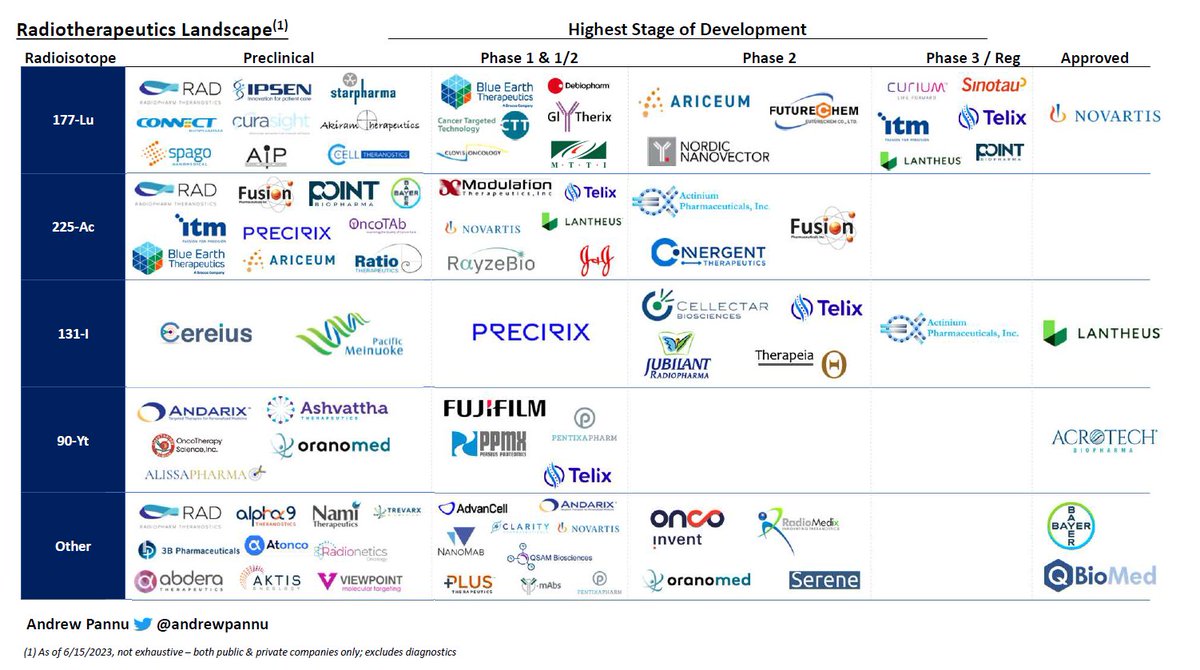
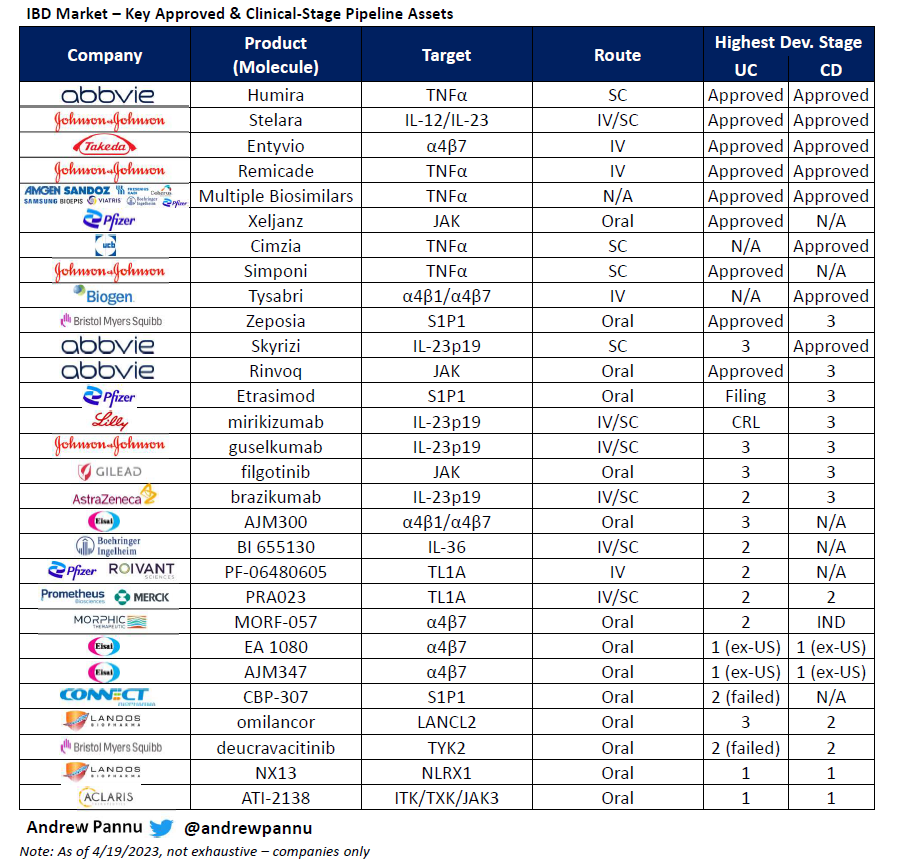
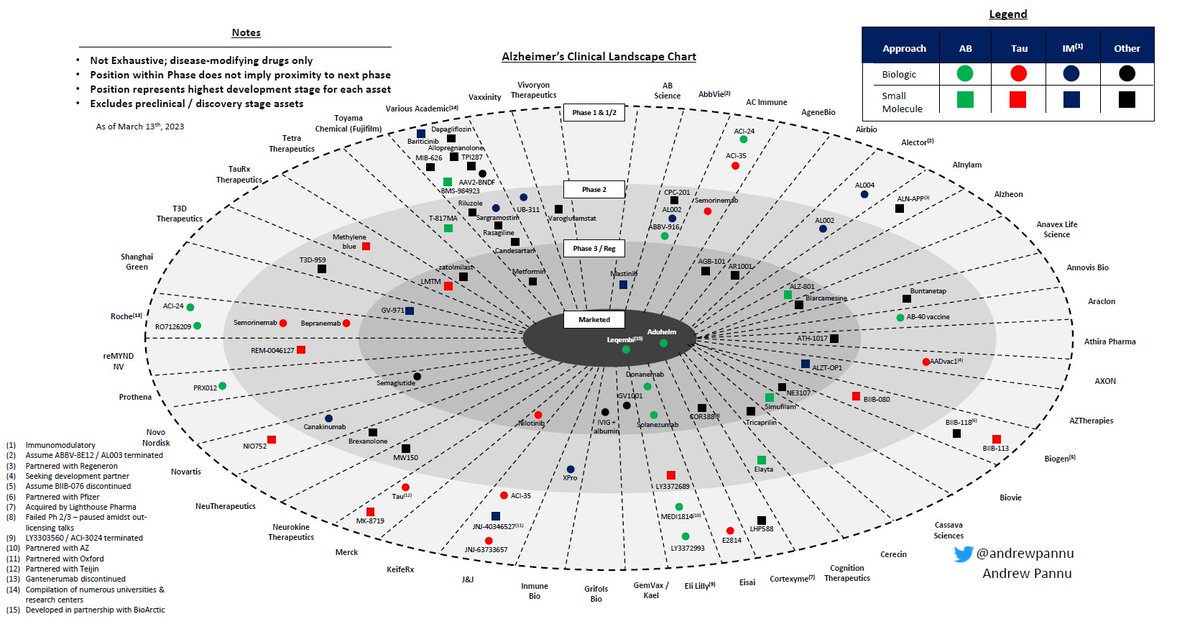
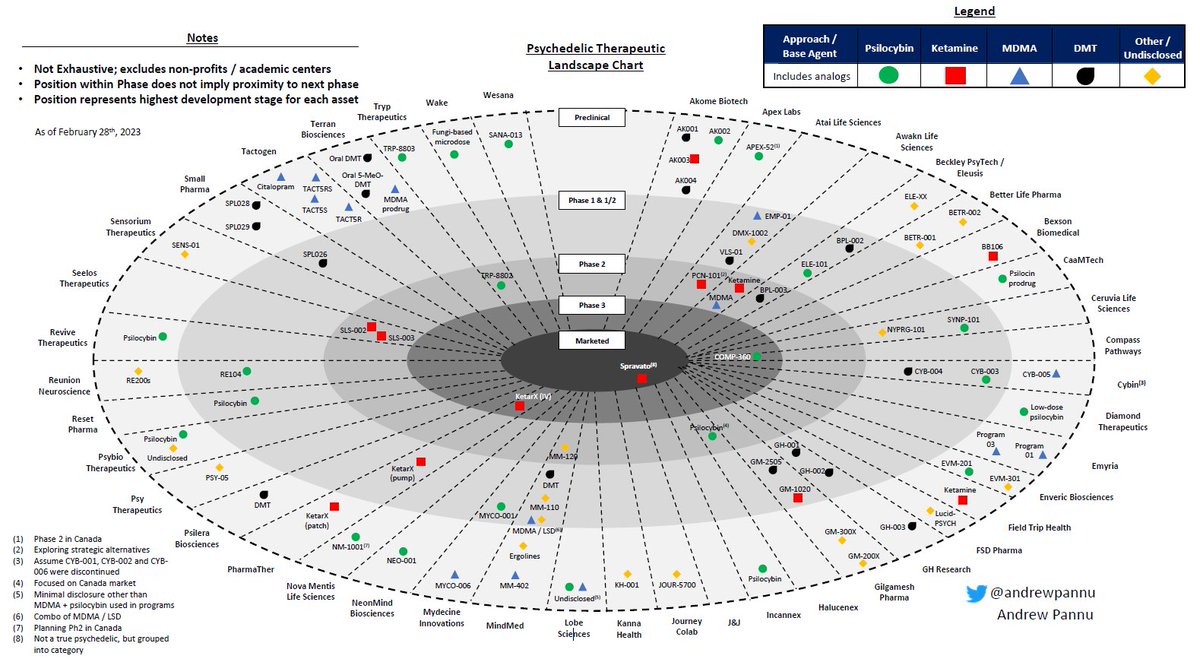
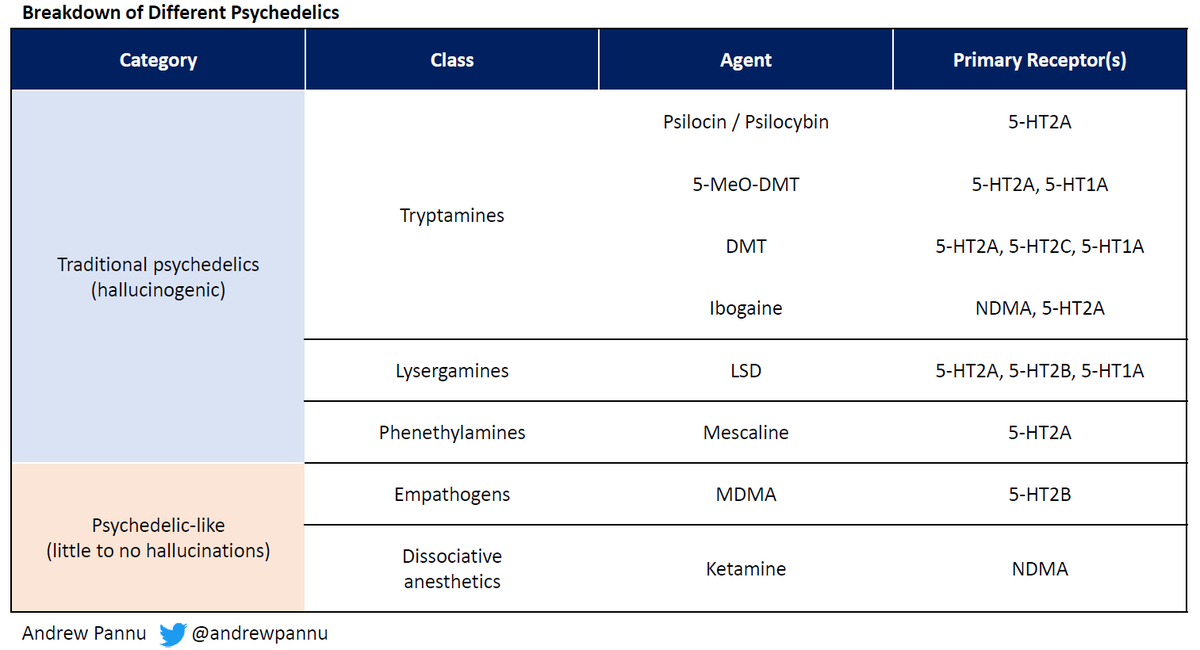
Refreshed obesity competitive landscape
Updated thoughts on this massive market following today's positive SELECT trial readout ($NVO) and Mounjaro sales beat ($LLY):
Updated thoughts on this massive market following today's positive SELECT trial readout ($NVO) and Mounjaro sales beat ($LLY):

Obesity refresher:
• Defined as BMI >30kg/m^2
• >750M patients globally, including ~40% of US adults --> expected to increase to 1B people / ~50% of US adults by 2030
• Linked to 1/5 US adult deaths
However, just ~2% of people (15M) are on anti-obesity medications (AOM)
• Defined as BMI >30kg/m^2
• >750M patients globally, including ~40% of US adults --> expected to increase to 1B people / ~50% of US adults by 2030
• Linked to 1/5 US adult deaths
However, just ~2% of people (15M) are on anti-obesity medications (AOM)

$NVO's SELECT trial was the most meaningful catalyst for this space in 2023 - it measured Wegovy's impact on reducing major adverse CV events (MACE) over a period of up to 5 years
The trial enrolled >17K overweight or obese adults with a history of CV disease & no diabetes
The trial enrolled >17K overweight or obese adults with a history of CV disease & no diabetes
Before today, KOLs considered a ~16% redux in MACE to be clinically meaningful - SELECT delivered a 20% redux
Things to monitor once the full data is released:
(1) Any separation in all-cause mortality
(2) What contributed to MACE redux
(3) Safety
+ readthrough to SURMOUNT-MMO
Things to monitor once the full data is released:
(1) Any separation in all-cause mortality
(2) What contributed to MACE redux
(3) Safety
+ readthrough to SURMOUNT-MMO
Based on these results, we can expect $NVO to file for a label indication expansion for Wegovy
Payors will see added pressure to reimburse these obesity drugs moving forward, given a redux in CV outcomes has massive downstream cost-saving implications
Payors will see added pressure to reimburse these obesity drugs moving forward, given a redux in CV outcomes has massive downstream cost-saving implications
Importantly, SELECT could pave the way to Medicare coverage of AOMs, which has been locked since 2003
Legislative amendments will take time, but this could potentially be a prominent healthcare issue during the 2024 election cycle, alongside the IRA
Legislative amendments will take time, but this could potentially be a prominent healthcare issue during the 2024 election cycle, alongside the IRA
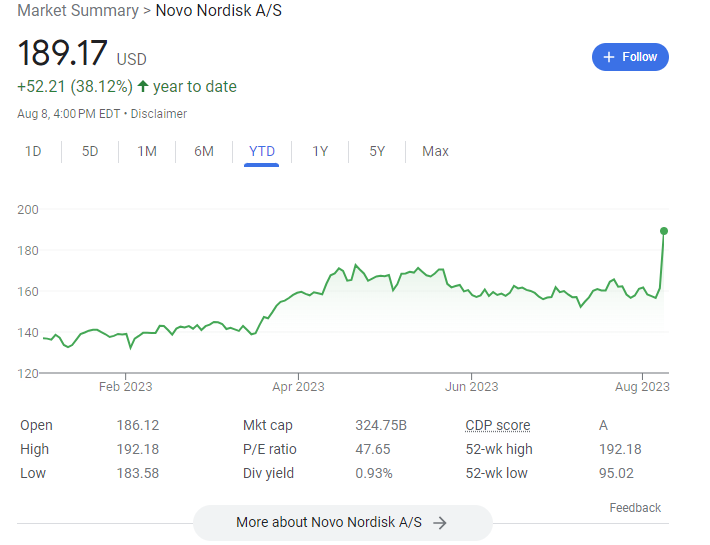
Separately, $LLY reported Q2 results and Mounjaro crushed expectations, with sales coming in >$200M above consensus estimates
Mounjaro is not yet approved for obesity, but off-label usage has been massive ($LLY previously reported ~33% of scripts were diabetes naive patients)
Mounjaro is not yet approved for obesity, but off-label usage has been massive ($LLY previously reported ~33% of scripts were diabetes naive patients)
That off-label usage has been driven by
(1) supply constraints with Wegovy and
(2) unprecedented demand, largely driven by social media (i.e. patient journeys) & pop culture (celebrity usage)
#Mounjaro, #Wegovy & #Ozempic have billions of views across notable platforms
(1) supply constraints with Wegovy and
(2) unprecedented demand, largely driven by social media (i.e. patient journeys) & pop culture (celebrity usage)
#Mounjaro, #Wegovy & #Ozempic have billions of views across notable platforms

Beyond Mounjaro, $LLY's Ph2 asset retatrutide demonstrated BIC efficacy of 24.2% mean weight reduction at 48 weeks (~58 lbs). This is in-line with bariatric surgery
Compare this to Wegovy (~12%) and Mounjaro (~16-22%) and the arms race may be just beginning
Compare this to Wegovy (~12%) and Mounjaro (~16-22%) and the arms race may be just beginning
Results for retatrutide may even be better than advertised, since:
(1) the trial enrolled a larger proportion of men than women, and women tend to lose a higher proportion of weight (~29% vs. ~22%)
(2) patients had yet to reach a plateau by study end, implying more potential
(1) the trial enrolled a larger proportion of men than women, and women tend to lose a higher proportion of weight (~29% vs. ~22%)
(2) patients had yet to reach a plateau by study end, implying more potential
All of this weight loss has translated into massive market share gains for $LLY and $NVO
$LLY is now the world's most valuable pharma company and collectively the two giants are worth >$825B


$LLY is now the world's most valuable pharma company and collectively the two giants are worth >$825B

Other companies are taking notice - as the space gets increasingly crowded, differentiation beyond efficacy & safety will be important:
• Dosing convenience (i.e. less frequent injections or oral)
• Weight loss kinetics (slope of weight loss)
• Data in sub-populations
• Dosing convenience (i.e. less frequent injections or oral)
• Weight loss kinetics (slope of weight loss)
• Data in sub-populations
This analysis has been focused on general (polygenic) obesity, but there is a separate pipeline for various genetically-driven obesities (monogenetic obesity)
One player in this space is $RYTM which launched Imcivree for patients with 3 genetic deficiencies (POMC, PCSK1, LEPR)
One player in this space is $RYTM which launched Imcivree for patients with 3 genetic deficiencies (POMC, PCSK1, LEPR)
That's all - If you enjoyed this, follow me
@andrewpannu for more biotech charts, musings and breakdowns
If you'd like a PDF of the landscape, you can find it here, along with all of my other reports & graphics: andrewpannu.com/downloads/
@andrewpannu for more biotech charts, musings and breakdowns
If you'd like a PDF of the landscape, you can find it here, along with all of my other reports & graphics: andrewpannu.com/downloads/
• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter