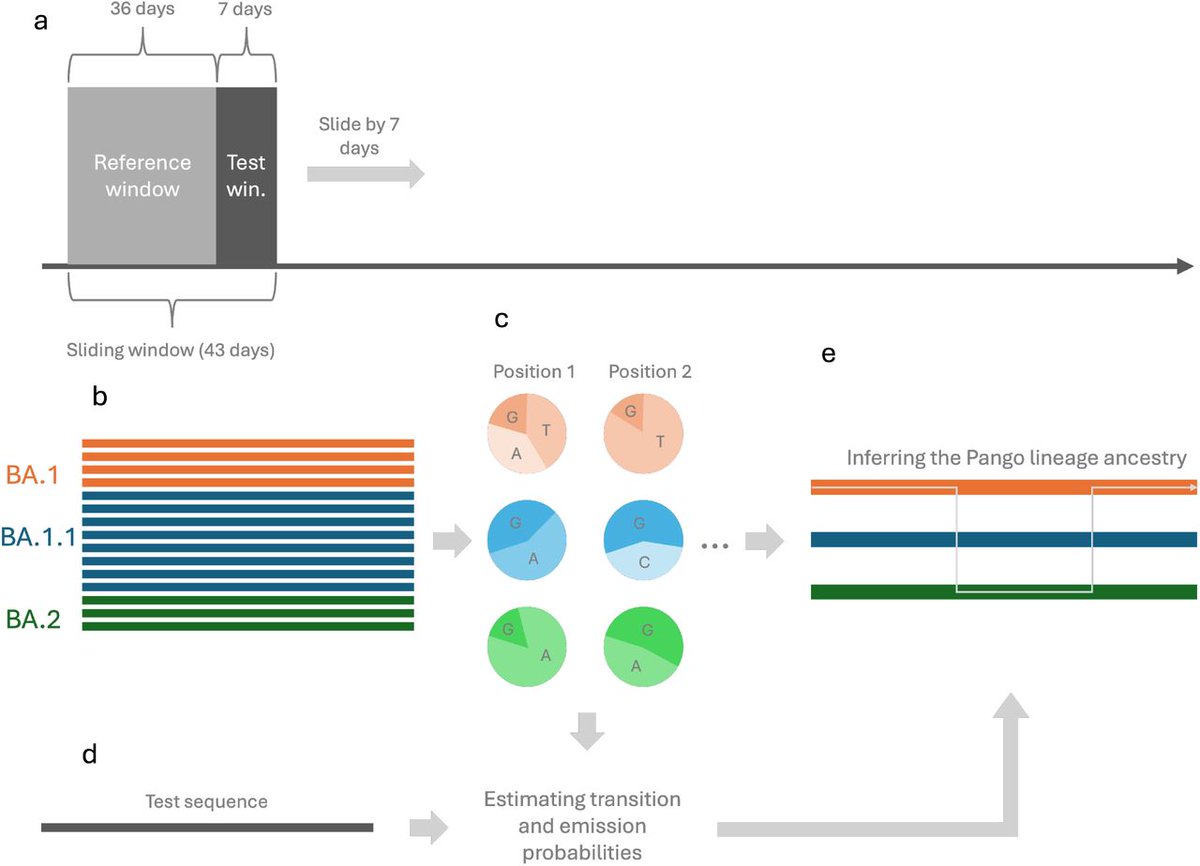
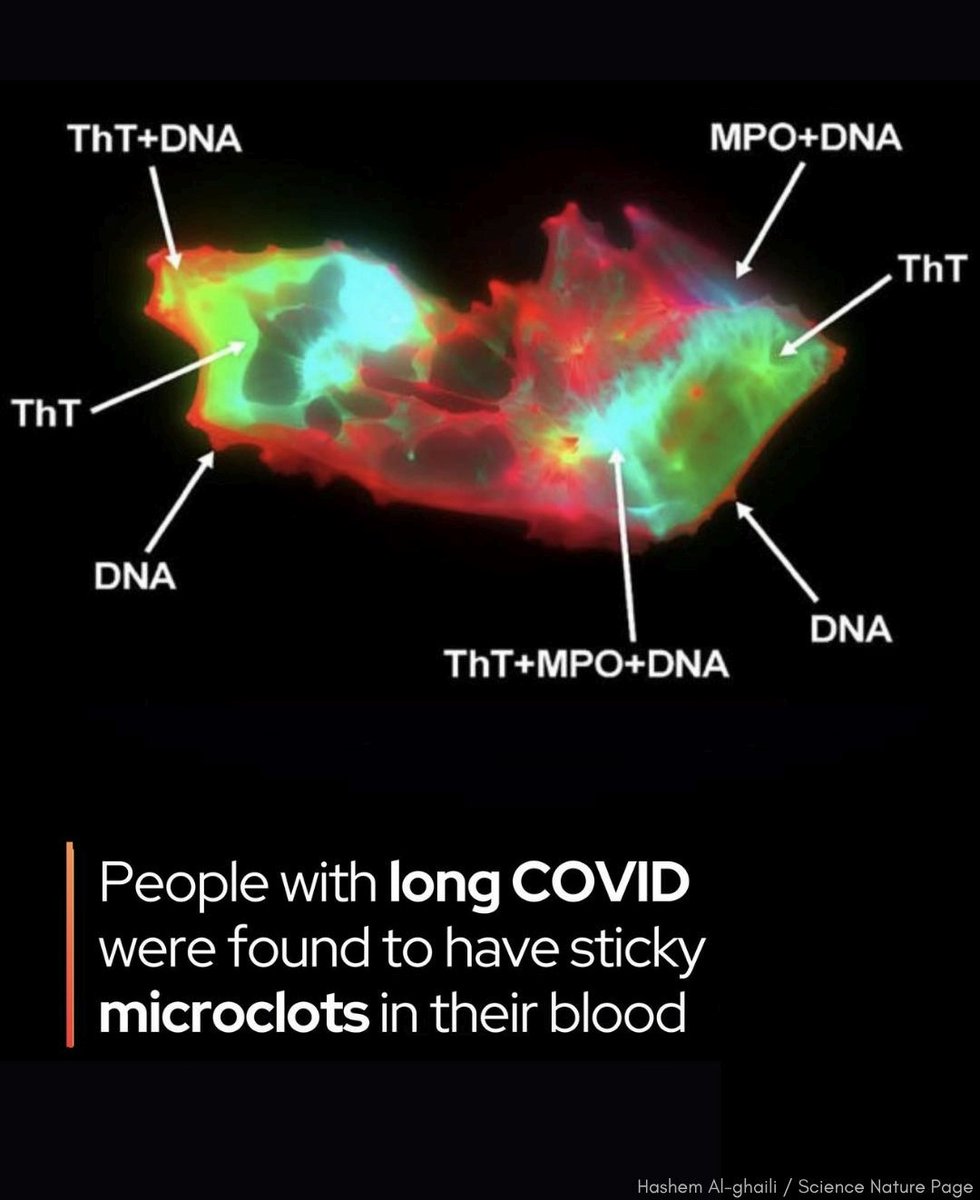
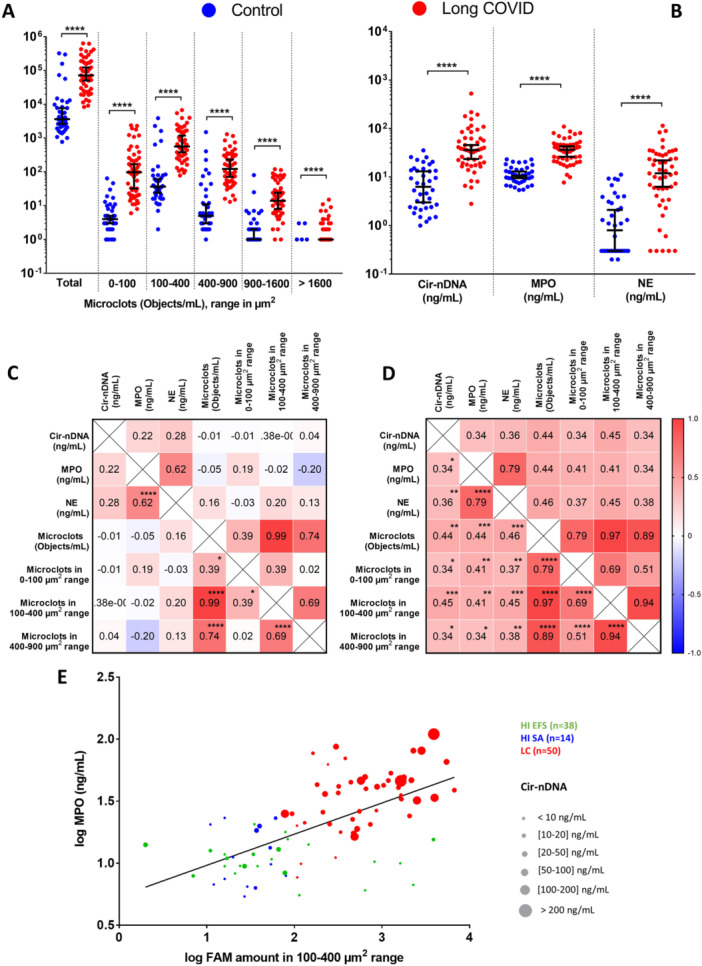
IMMUNE IMPRINTING !
It is strange that almost no one (except @MarionKoopmans) has talked about the latest study by @yunlong_cao and his colleagues, on this subject 🤔
It is strange that almost no one (except @MarionKoopmans) has talked about the latest study by @yunlong_cao and his colleagues, on this subject 🤔

2) "Repeated Omicron exposures override ancestral SARS-CoV-2 immune imprinting"
Key findings :
▶️ "Immune imprinting induced by wildtype (WT)-based vaccination would compromise the antibody response to Omicron-based boosters"biorxiv.org/content/10.110…
Key findings :
▶️ "Immune imprinting induced by wildtype (WT)-based vaccination would compromise the antibody response to Omicron-based boosters"biorxiv.org/content/10.110…
3) ▶️ Single Omicron-boosting is heavily limited by immune imprinting, especially when using variants antigenically distinct from WT, like XBB"
▶️ "Repeated Omicron infections could also alleviate WT-vaccination-induced immune imprinting and generate high neutralizing titers ...
▶️ "Repeated Omicron infections could also alleviate WT-vaccination-induced immune imprinting and generate high neutralizing titers ...
4) ...against XBB.1.5 and XBB.1.16"
▶️ "Our findings suggest the WT component should be abandoned when updating COVID-19 vaccine antigen compositions to XBB lineages, and those who haven’t been exposed to Omicron yet should receive two updated vaccine boosters."
▶️ "Our findings suggest the WT component should be abandoned when updating COVID-19 vaccine antigen compositions to XBB lineages, and those who haven’t been exposed to Omicron yet should receive two updated vaccine boosters."
11) Thanks for reading 🙏 and let see the reaction of the experts on this subject 😁
FYI
@LongDesertTrain @siamosolocani @SystemsVirology
@AltenbergLee
@mrmickme
@DavidJoffe64 @HarrySpoelstra
@UseBy2022 @outbreakupdates @arijitchakrav
@C_A_Gustave
@UseBy2022
@vipintukur
FYI
@LongDesertTrain @siamosolocani @SystemsVirology
@AltenbergLee
@mrmickme
@DavidJoffe64 @HarrySpoelstra
@UseBy2022 @outbreakupdates @arijitchakrav
@C_A_Gustave
@UseBy2022
@vipintukur
• • •
Missing some Tweet in this thread? You can try to
force a refresh