Does SARS-CoV-2 have the potential to cause cancers?
What appears to be SARS-CoV-2’s ability to sometimes incite chronic inflammation. Cancerous viruses usually establish persistent long-term infections in their host, and they’re good at hiding from the immune system. 1/
What appears to be SARS-CoV-2’s ability to sometimes incite chronic inflammation. Cancerous viruses usually establish persistent long-term infections in their host, and they’re good at hiding from the immune system. 1/
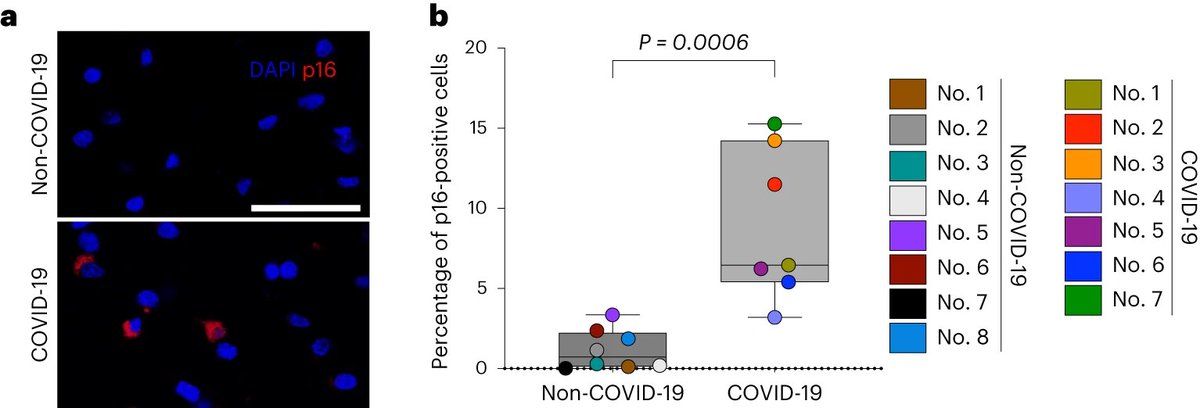
Further, emerging evidence supports the idea that some people harbor “viral reservoirs,” places in the body where perhaps SARS-CoV-2 or some fragment of the virus might persist. 2/
The viral reservoir could be replicating the virus (we don’t have evidence of that in humans, but it was detected in infected macaques), or pieces of viral RNA could be producing proteins or be lying dormant. 3/
Even if the remaining pieces of the virus are not infectious, researchers hypothesize that their presence may still be able to alter people’s immune responses in damaging ways. 4/
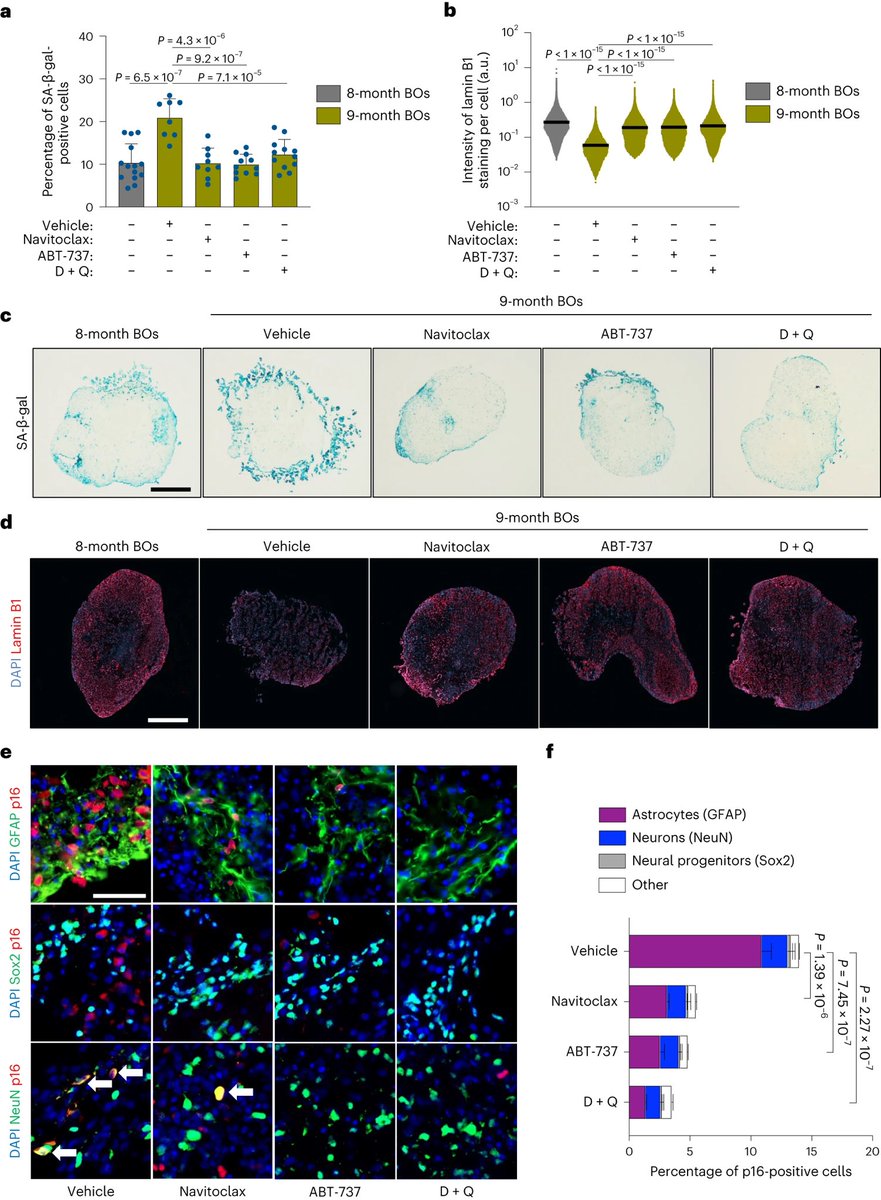
But while we know SARS-CoV-2 RNA persists in many tissues, it’s completely uncertain whether that drives the kinds of chronic inflammation that could lead to cancer. 5/
Researchers and experts are watching closely for that next surprise. “Even if you imagine that 1% of the people with persistent infection would develop a cancer, the number is huge,” 6/
Hopefully it’s not even 1%. It’s like 0%. That’s why we should run some experiments–just to be sure that nothing’s going to happen. Just to be on the safe side. 7/
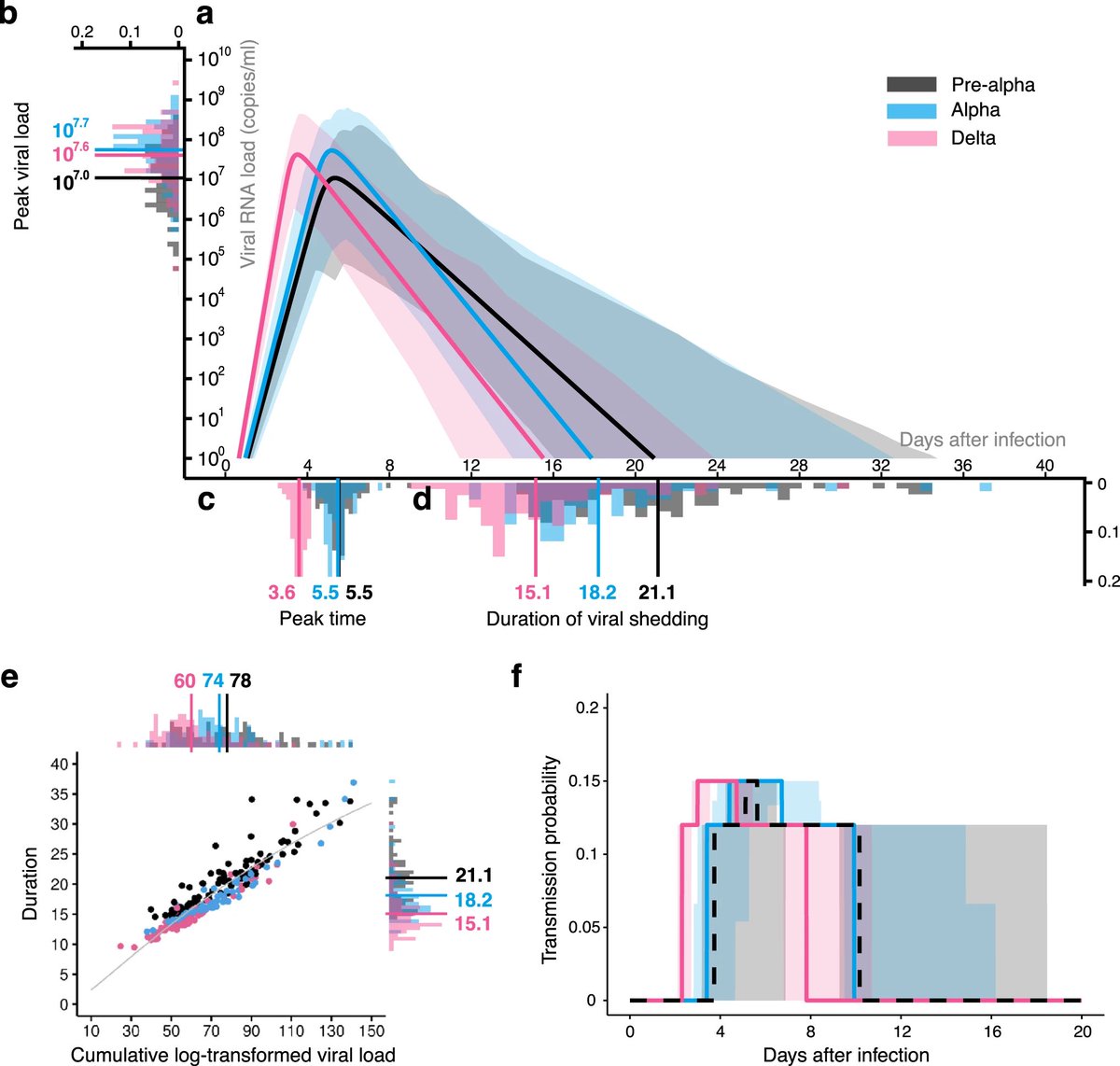
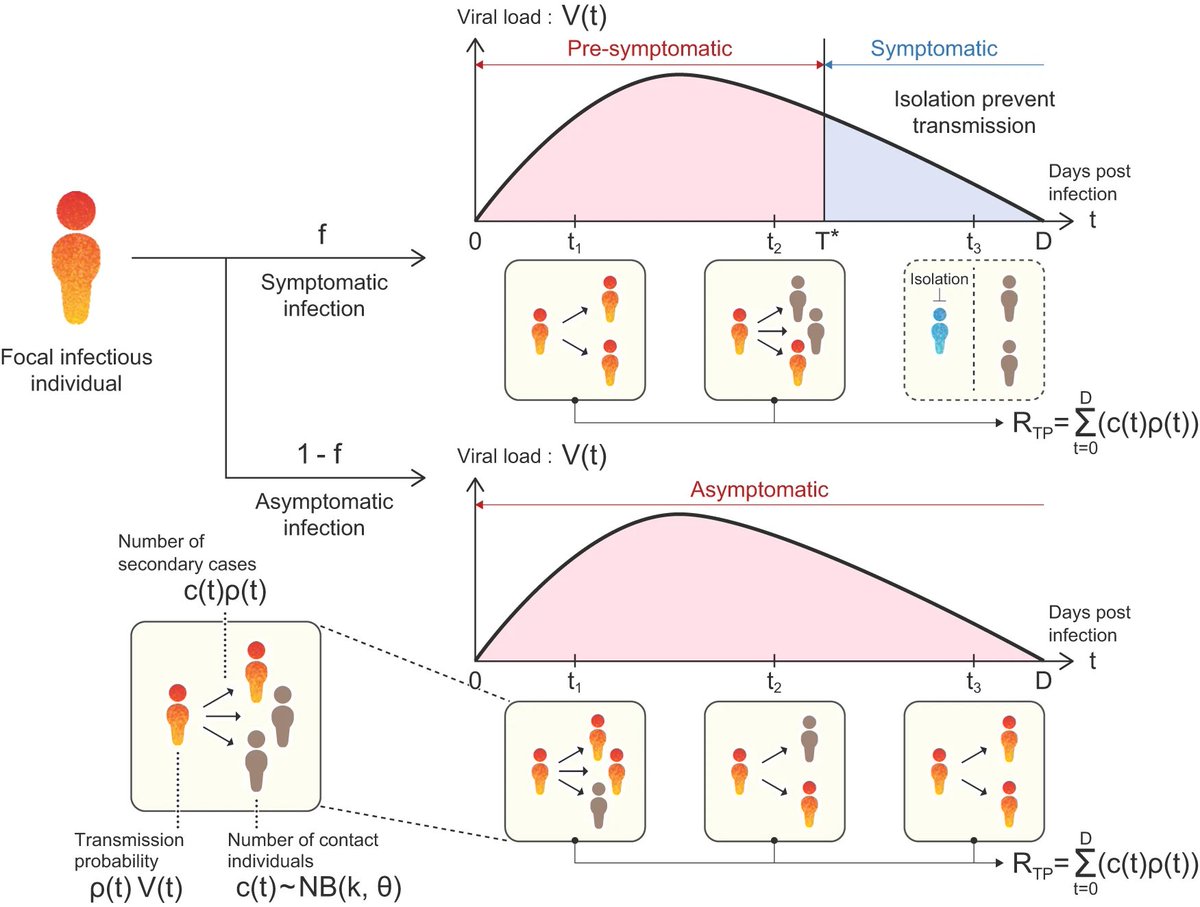
Viral infections are thought to be responsible for about 15%-20% of cancer cases globally. And on sheer scale alone–>770 million cases worldwide, nearly 7 million deaths, and the ability of some strains to transmit efficiently and evade vaccine- or infection-acquired immunity 8/
“There’s not great evidence to support SARS-CoV-2 being an oncogenic virus–but this virus has surprised us over and over.”
SARS-CoV-2 has proven to be a formidable, if yet mysterious, pathogen. We just don’t know yet what this virus is capable of doing. 9/
SARS-CoV-2 has proven to be a formidable, if yet mysterious, pathogen. We just don’t know yet what this virus is capable of doing. 9/
Here is the link to the full article analysing the oncogenic potential of the SARS-CoV-2 with some great inputs by @VirusesImmunity 👇 10/10
fortune.com/2023/11/23/ins…
fortune.com/2023/11/23/ins…
• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter