1/ 🧵🚨🧬💉 "turbo" cancer
Selective Advantage Mutations to Tumors Path:
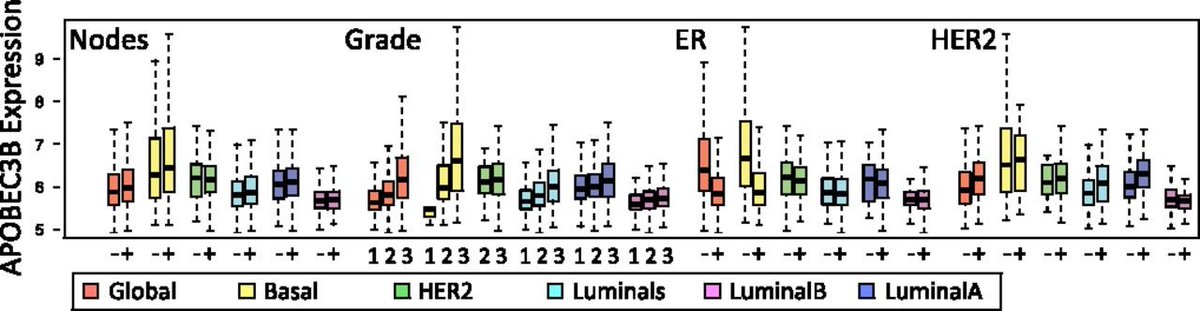
Somatic mutagenesis: APOBEC3B is a cellular deaminase--overexpressed in cancers and a cause of cancer-associated mutations.
APOBEC3B/3G binds to dsDNA
(HEAVY SCIENCE)
(maybe I'm wrong-- just a ginger)
Selective Advantage Mutations to Tumors Path:
Somatic mutagenesis: APOBEC3B is a cellular deaminase--overexpressed in cancers and a cause of cancer-associated mutations.
APOBEC3B/3G binds to dsDNA
(HEAVY SCIENCE)
(maybe I'm wrong-- just a ginger)
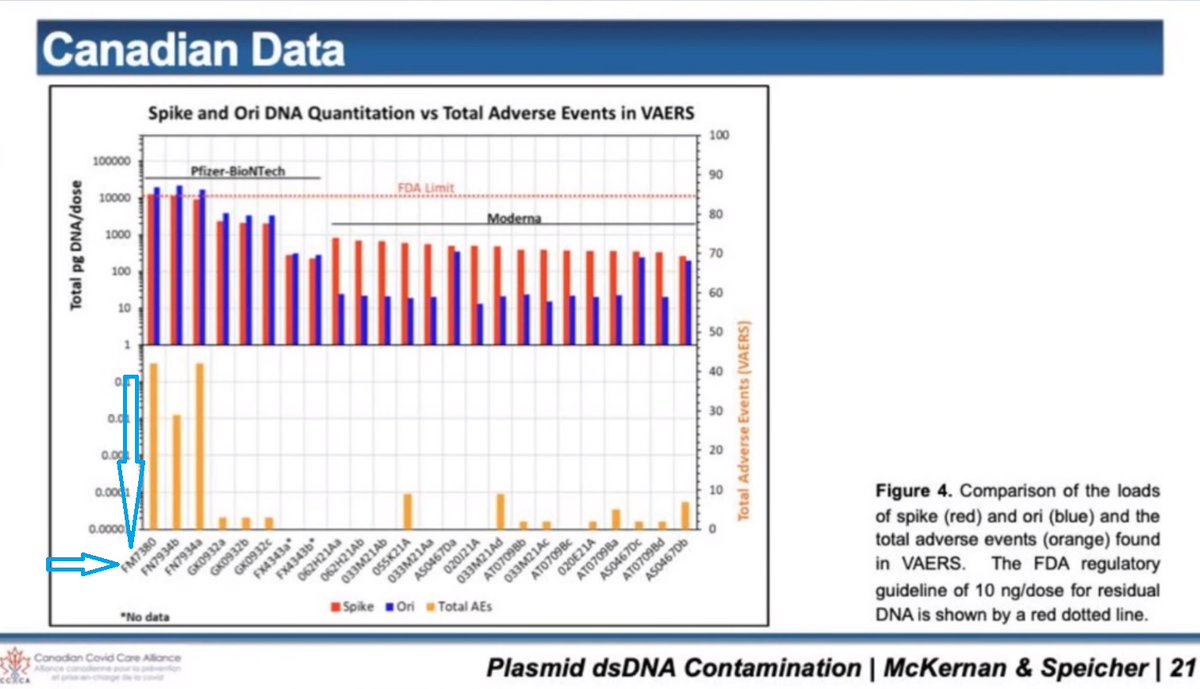
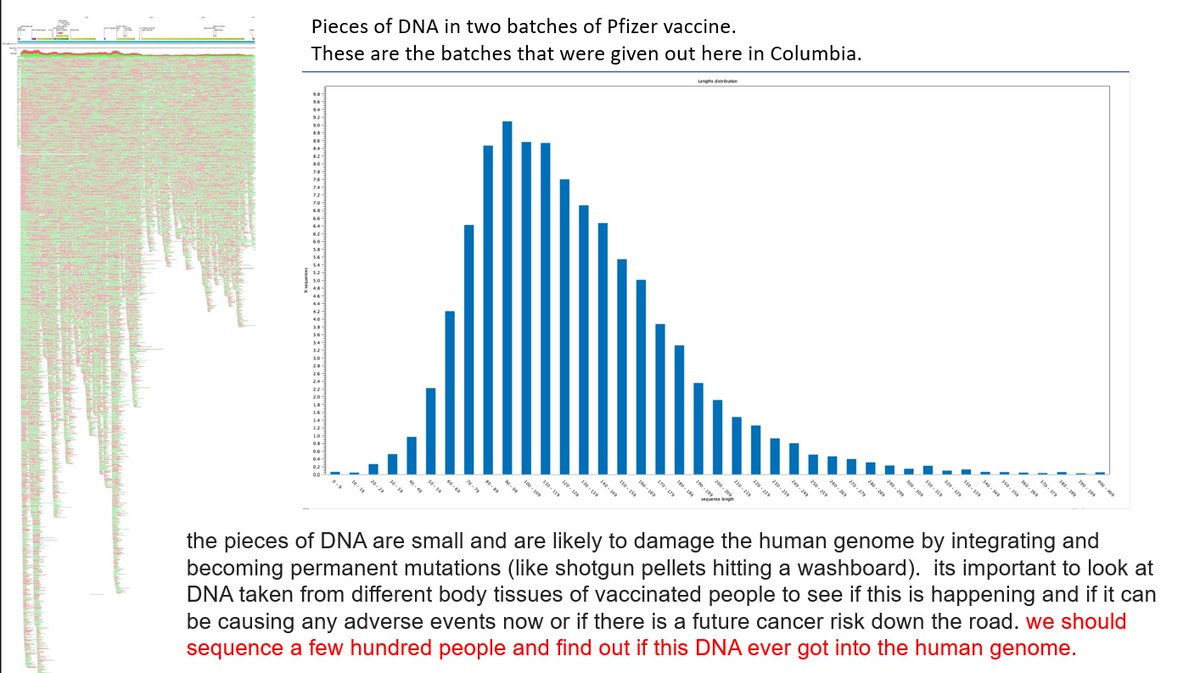
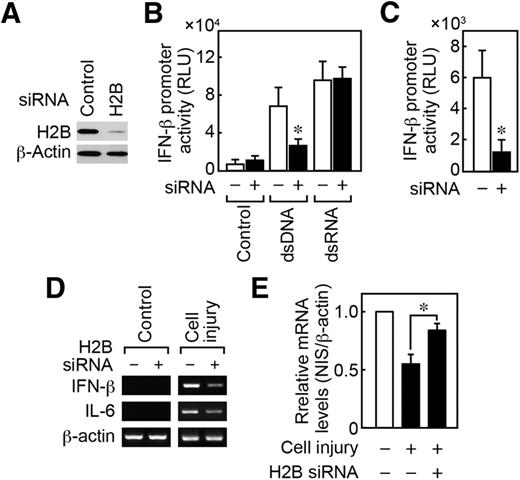
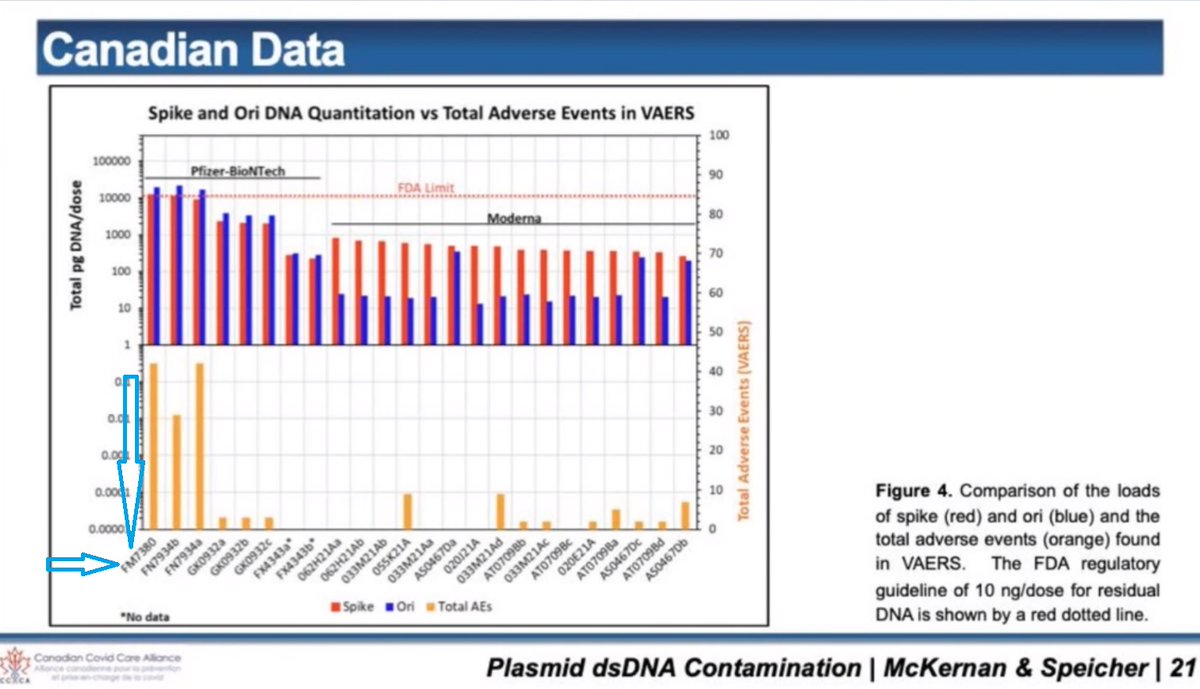
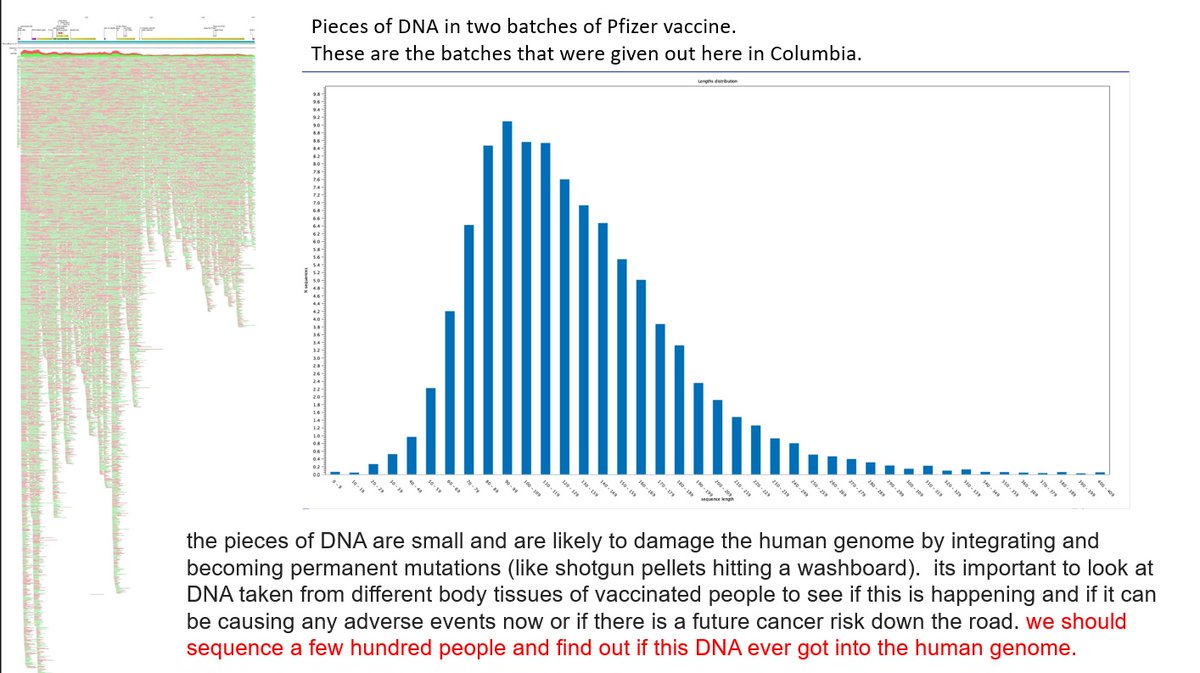
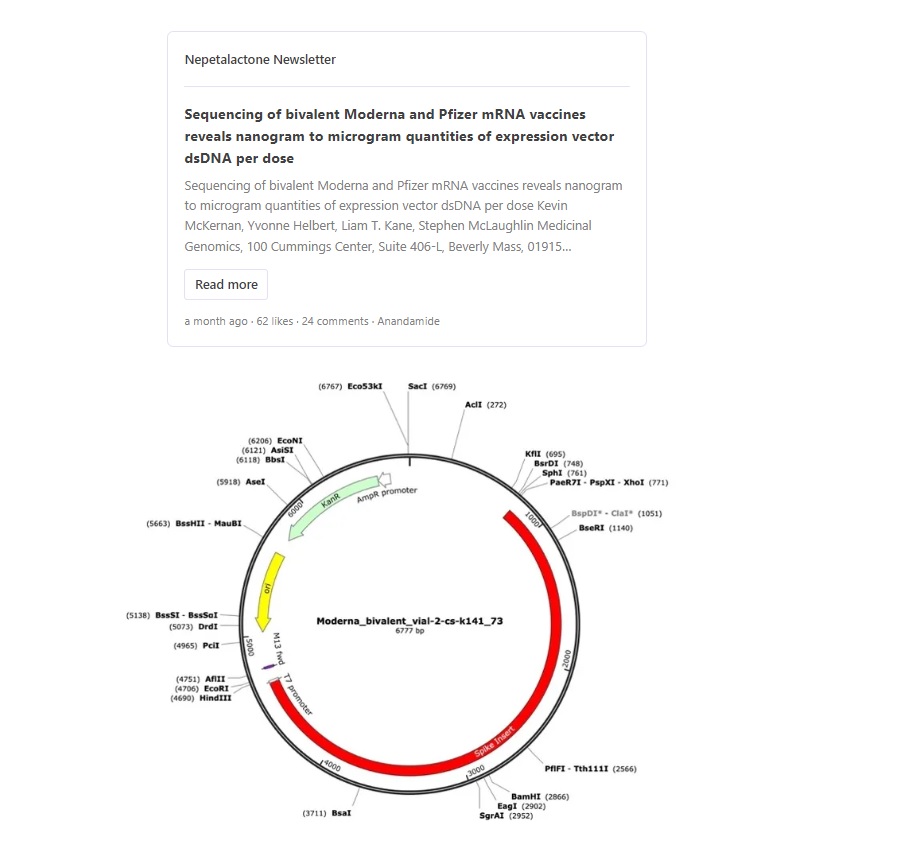
2/ DNA plasmid contamination was recently found in vials of cov!d modRNA "vaccines". DNA plasmid is dsDNA. That is double stranded DNA. There is such a thing as ssDNA. That is single stranded DNA.
DNA plasmid contains what is called a CpG motif.
DNA plasmid contains what is called a CpG motif.
https://twitter.com/_HeartofGrace_/status/1706387728286269883
3/ There are different types of APOBEC. APOBEC3A (A3A), is a prominent source of mutations in multiple cancer types, but it prefers ssDNA. That is single stranded DNA. It is not a fan of dsDNA. (I am not going to explain what APOBEC is here)
nature.com/articles/s4159…

nature.com/articles/s4159…

4/ The two main types of the APOBEC family that have an affinity to dsDNA (they also will interact with ssDNA) are APOBEC3B/APOBEC3G.
There have been talk about R loops and things like that, but that SHOULDN'T occur here (maybe I will make another thread on why )
There have been talk about R loops and things like that, but that SHOULDN'T occur here (maybe I will make another thread on why )
5/ APOBEC3G (A3G):
CpG Motif Binding: A3G has been reported to have a preference for binding to DNA containing CpG motifs. CpG motifs are regions of DNA where a cytosine is followed by a guanine. A3G has been shown to bind to these sequences.
The primary enzymatic activity of
CpG Motif Binding: A3G has been reported to have a preference for binding to DNA containing CpG motifs. CpG motifs are regions of DNA where a cytosine is followed by a guanine. A3G has been shown to bind to these sequences.
The primary enzymatic activity of
6/ A3G is cytidine deamination. It catalyzes the conversion of cytidine to uridine in single-stranded DNA.
APOBEC3B (A3B) exhibits cytidine deaminase activity on double-stranded DNA (dsDNA), and its mutagenic not just in single-stranded DNA (ssDNA).
APOBEC3B (A3B) exhibits cytidine deaminase activity on double-stranded DNA (dsDNA), and its mutagenic not just in single-stranded DNA (ssDNA).
7/ A3B has a preference for binding to DNA with a preference for CpG motifs, and its enzymatic activity induces C-to-U transitions in both strands of DNA.
When the A3B comes in contact in the cell with dsDNA plasmid pieces, what could occur, is it would deaminate cytidine
When the A3B comes in contact in the cell with dsDNA plasmid pieces, what could occur, is it would deaminate cytidine
8/ residues in both strands of double-stranded DNA of the DNA plasmid contamination.
A3B SHOULD induce C-to-U transitions in both strands of linearized dsDNA. The key factor influencing A3B activity, is its preference for CpG motifs rather than the three-dimensional structure
A3B SHOULD induce C-to-U transitions in both strands of linearized dsDNA. The key factor influencing A3B activity, is its preference for CpG motifs rather than the three-dimensional structure
9/ of the DNA found in cells, but go after the linearized pieces of DNA plasmid.
To reiterate CpG motifs are regions where a cytosine nucleotide is followed by a guanine nucleotide in the DNA plasmid.
My understanding of what this means, as a tall ginger on twitter is:
To reiterate CpG motifs are regions where a cytosine nucleotide is followed by a guanine nucleotide in the DNA plasmid.
My understanding of what this means, as a tall ginger on twitter is:
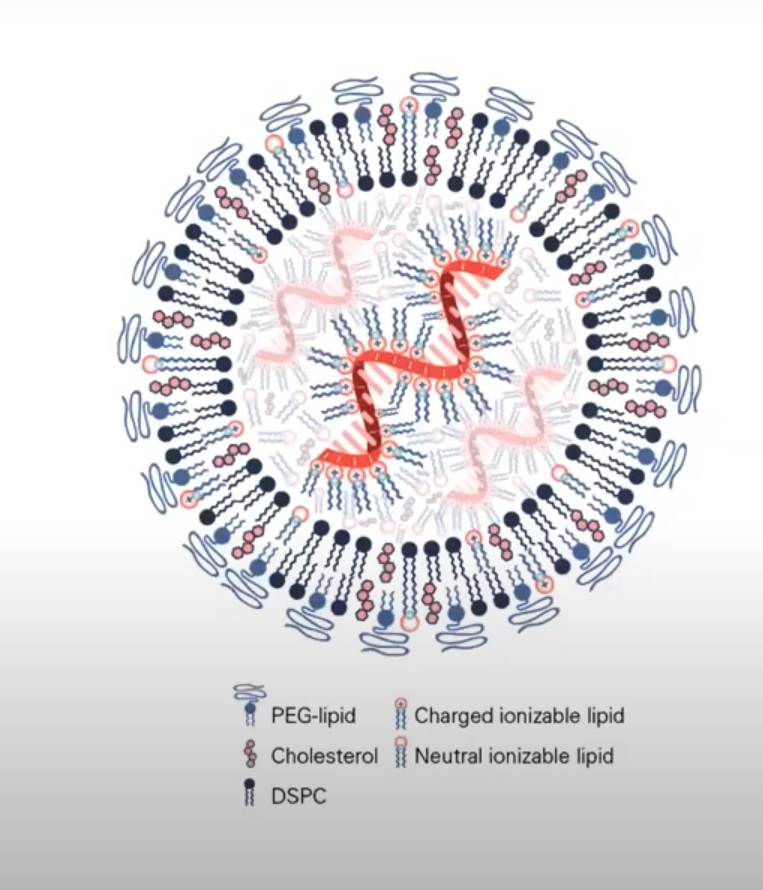
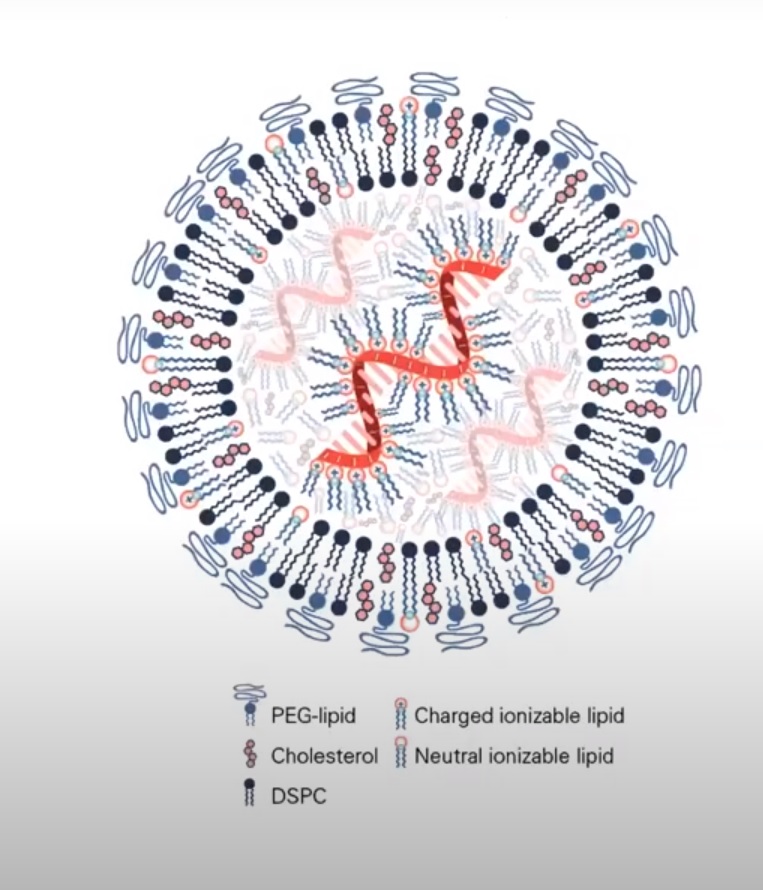
10: A3B-induced cytidine deamination on both strands of dsDNA results in the formation of uracil-guanine mismatches. This means a mutagenic effect occurs on both strands of the CpG motif. This would happen of course, before it got pulled into the nucleus. If the LNP opened up


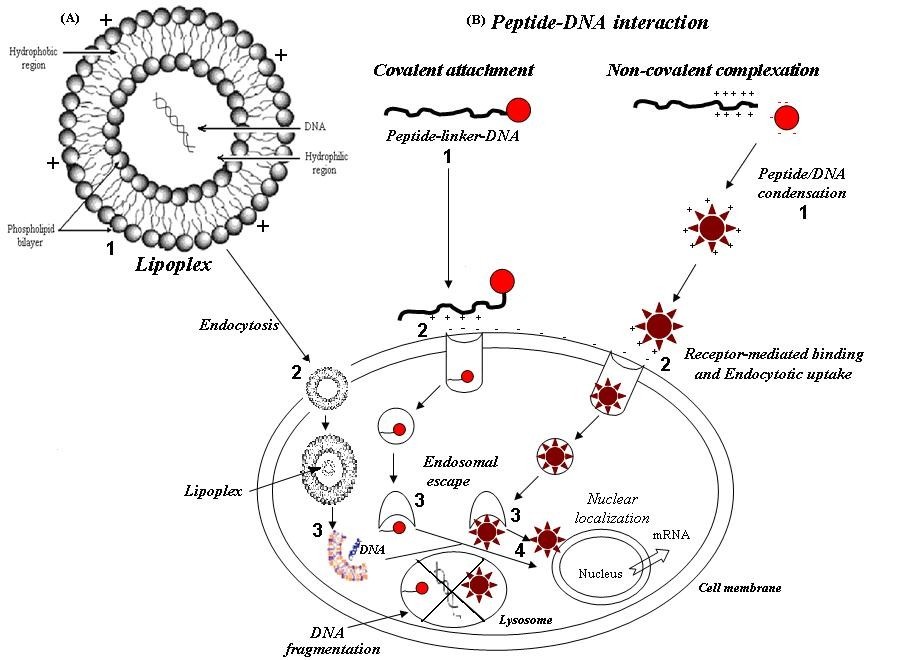
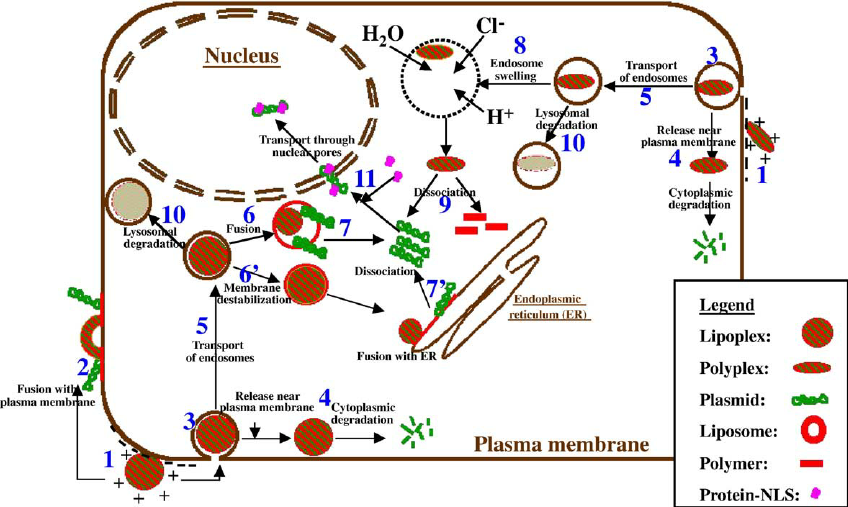
11/ And if LNP is combo of DNA/RNA hybrid, first LNP would open, RNA head to ribosome, then, DNA plasmid should be acted upon by APOBEC3B and/or STING pathway.
If APOBEC3B grabs it:
(A3B where its overexpression is associated with an increased mutation load in cancer genomes.
If APOBEC3B grabs it:
(A3B where its overexpression is associated with an increased mutation load in cancer genomes.
12/then A3B, when activated in response to exogenous DNA, induces mutations in foreign plasmid DNA.
This mutagenic activity includes the deamination of cytidines, and uracil creation.
If exogenous DNA integrates into genome, the A3B-induced mutations become part of genome.
This mutagenic activity includes the deamination of cytidines, and uracil creation.
If exogenous DNA integrates into genome, the A3B-induced mutations become part of genome.
13/ it is BAD enough that the CpG motifs, SV40, and other parts of the DNA plasmid could have impacts, but in this scenario, the CpG motif is not going to stay intact. It will not be just a CpG entering the mix. It will now be integrated pieces of UcG. We now have integration
14/ of UcG, uracil c guanine. Of course, depending on the location and nature of these mutations, they may lead to inactivation of tumor suppressor genes or the activation of proto-oncogenes.
But, now we have uracil in there where it does not belong. This can lead to :
But, now we have uracil in there where it does not belong. This can lead to :
15/ Selective Advantage:
(Maybe):
Cells with integrated exogenous DNA carrying A3B-induced mutations may have a selective advantage over other cells.
The mutations induced by A3B might confer certain benefits to the cells, such as improved survival, enhanced growth, or resistance
(Maybe):
Cells with integrated exogenous DNA carrying A3B-induced mutations may have a selective advantage over other cells.
The mutations induced by A3B might confer certain benefits to the cells, such as improved survival, enhanced growth, or resistance
16/ to specific conditions.
This could lead to clonal expansion: proliferation and growth of a population of cells that are derived from a single parent cell, from the cells with integrated exogenous DNA and A3B-induced mutations that now have a growth advantage over other cells.
This could lead to clonal expansion: proliferation and growth of a population of cells that are derived from a single parent cell, from the cells with integrated exogenous DNA and A3B-induced mutations that now have a growth advantage over other cells.
17/ 🚨 ("turbo cancer")
These mutated cells undergo repeated divisions, leading to the formation of a population or clone of cells with similar genetic alterations.
These mutated cells undergo repeated divisions, leading to the formation of a population or clone of cells with similar genetic alterations.
18/ The clonal expansion of cells with integrated exogenous DNA and A3B-induced mutations may create a tumor.
Tumor formation involves the uncontrolled and abnormal growth of cells, and the clonal expansion of mutated cells is a fundamental process in the development of tumors.
Tumor formation involves the uncontrolled and abnormal growth of cells, and the clonal expansion of mutated cells is a fundamental process in the development of tumors.
19/ APOBEC3B (A3B)-induced mutations provide a significant growth advantage, it may contribute to faster clonal expansion and tumor development.
specific mutations induced by A3B and their impact on key regulatory genes or pathways can influence the speed of tumor formation.
specific mutations induced by A3B and their impact on key regulatory genes or pathways can influence the speed of tumor formation.
20/ Certain mutations may drive more aggressive and rapid tumor growth.
The extent of the selective advantage conferred by A3B-induced mutations will play a role. If these mutations provide a significant growth advantage, it may contribute to faster clonal expansion and tumor
The extent of the selective advantage conferred by A3B-induced mutations will play a role. If these mutations provide a significant growth advantage, it may contribute to faster clonal expansion and tumor
21/ development.
The presence of additional mutations in other genes that cooperate with A3B-induced mutations can accelerate tumorigenesis. Certain combinations of mutations may drive rapid cancer.
The tissue or organ where the integration and mutations occur influence pace of
The presence of additional mutations in other genes that cooperate with A3B-induced mutations can accelerate tumorigenesis. Certain combinations of mutations may drive rapid cancer.
The tissue or organ where the integration and mutations occur influence pace of
22/ tumor development. Some tissues may be more permissive to rapid tumor growth than others.
f the immune system effectively recognizes and eliminates cells with the integrated exogenous DNA and A3B-induced mutations, it may slow down or prevent tumor formation.
f the immune system effectively recognizes and eliminates cells with the integrated exogenous DNA and A3B-induced mutations, it may slow down or prevent tumor formation.
23/ If A3B-induced mutations contribute to high levels of genomic instability, it may facilitate the accumulation of additional mutations, potentially accelerating tumor progression.
.nature.com/articles/s4159…
pnas.org/doi/10.1073/pn…
.nature.com/articles/s4159…
pnas.org/doi/10.1073/pn…
24/ Molecular mechanism and clinical impact of APOBEC3B-catalyzed mutagenesis in breast cancer
ncbi.nlm.nih.gov/pmc/articles/P…
nature.com/articles/s4158…
ncbi.nlm.nih.gov/pmc/articles/P…
nature.com/articles/s4158…
@P_J_Buckhaults
• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter