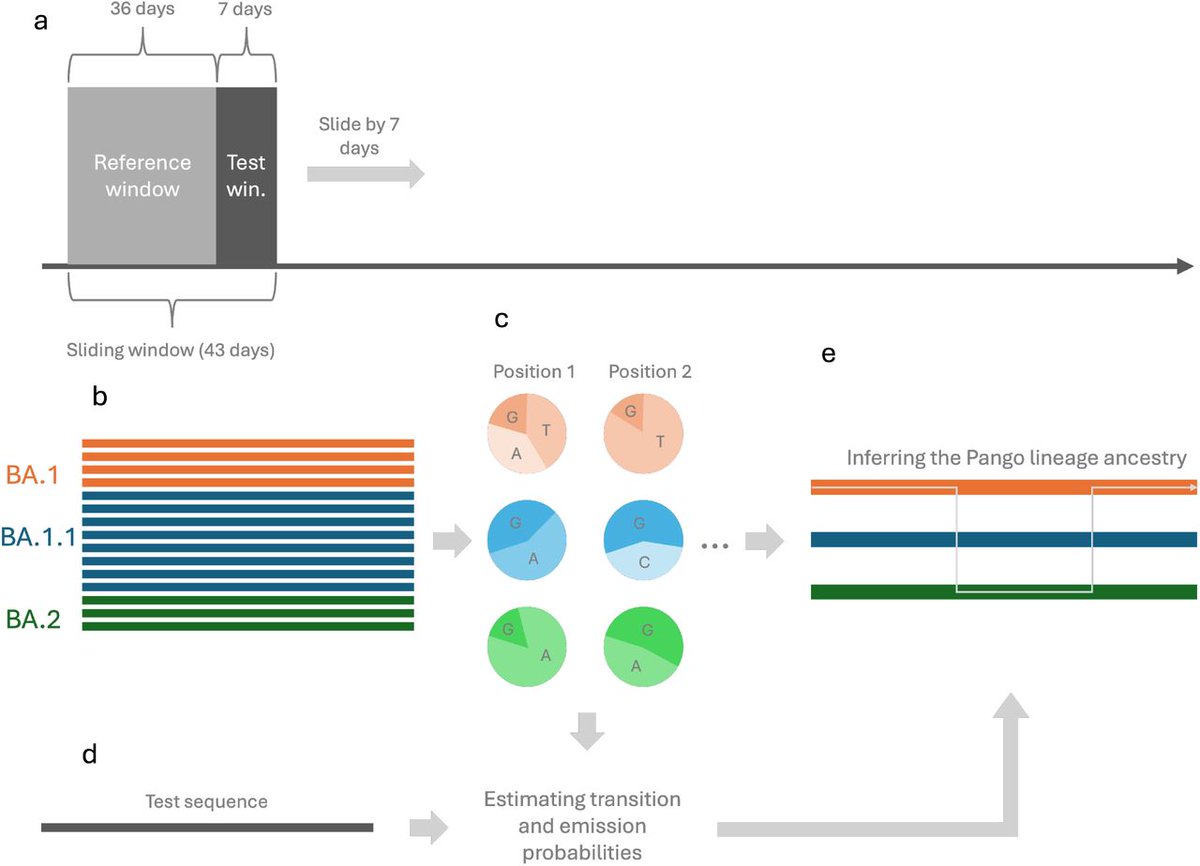
QUESTION to the VIRUS :
"You prefer to fuse with the cells, or to resist to interferons and to antibodies ?"
The virus : "I want both !"
"You prefer to fuse with the cells, or to resist to interferons and to antibodies ?"
The virus : "I want both !"
2) This virus never ceases to surprise us. Before discussing a fascinating study on this interplay between fusogenicity and immune escape, a short clarification.
SYNCYTIA: Syncytia are structures formed by the fusion of multiple cells ...
SYNCYTIA: Syncytia are structures formed by the fusion of multiple cells ...

3) ... into a single, multinucleated entity. This formation induce the fusion of infected cells with neighboring uninfected cells, leading to the formation of syncytia.
This allows the virus to spread more efficiently within the host and evade immune detection.
This allows the virus to spread more efficiently within the host and evade immune detection.

4) In this study,
they showed that "syncytia formation provides resistance to interferons and decreases antibody virus neutralization activity in cultured cells"
The researchers investigated also, "how the evolution of SARS-CoV-2 over timebiorxiv.org/content/10.110…
they showed that "syncytia formation provides resistance to interferons and decreases antibody virus neutralization activity in cultured cells"
The researchers investigated also, "how the evolution of SARS-CoV-2 over timebiorxiv.org/content/10.110…
5) .. affected the fusogenicity of the S protein. They measured syncytia formation and assessed the number of syncytia, syncytial area, and fluorescent intensity as indicators of fusogenicity. The results showed variations in fusogenicity among different strains, 

6) ...with an overall increase in fusogenicity as the virus evolved from the WA1 strain to the Delta variants. The original Omicron strain (BA.1) exhibited a decrease in fusogenicity compared to WA1, but there was a gradual increase in fusogenicity 

7) with subsequent Omicron variants (BA.2, BA4/5, BQ.1 to XBB)."
The question is to know also, if there is a link between these gains or decreases in fusogenicity and therefore via the syncytia of more or less resistance to interferons and antibodies
The question is to know also, if there is a link between these gains or decreases in fusogenicity and therefore via the syncytia of more or less resistance to interferons and antibodies

8) ... and the mutations on the Spike allowing gain in immune escape.
Question which remains open.
Thanks for reading 🙏
Question which remains open.
Thanks for reading 🙏

• • •
Missing some Tweet in this thread? You can try to
force a refresh