Australian #cultivatedmeat company @itsjustvow's safety info for its product was recently released, making it the 3rd safety dossier available to the public
The product is not yet approved & public comment is open until Feb 5th
Key info in this thread:
vegconomist.com/cultivated-cel…
The product is not yet approved & public comment is open until Feb 5th
Key info in this thread:
vegconomist.com/cultivated-cel…
@itsjustvow Let's start w/ the conclusion from the regulator Food Standards Australia New Zealand (FSANZ), which notes that key safety considerations such as allergenicity, toxicity of inputs, and microbiological risks were low. It is deemed safe to use as an ingredient in food.




@itsjustvow Cells: The cells used for production are an embryonic fibroblast line derived from Japanese quail.
The cells were originally obtained from an unnamed public repository based in Europe and adapted for use in production.
The cells were originally obtained from an unnamed public repository based in Europe and adapted for use in production.
Cells: The cells were spontaneously immortalized. The cells are not engineered or genetically modified.
No, immortalized cell lines are not the same thing as "eating cancer"
No, immortalized cell lines are not the same thing as "eating cancer"
https://x.com/elliotswartz/status/1625559396704215040?s=20
Media: The cells to produce the product are grown in a media that does not contain any antibiotics.
This is consistent with our expectations for the industry. Decoupling meat production from antibiotics is a huge benefit of #cultivatedmeat.
gfi.org/blog/cultivati…
This is consistent with our expectations for the industry. Decoupling meat production from antibiotics is a huge benefit of #cultivatedmeat.
gfi.org/blog/cultivati…
Media: The cells grow in suspension in a serum-free media.
Yes, as I've said for many years, the use of fetal bovine serum (FBS) is not a real bottleneck for the industry. Expect other products to follow. The amount of CM that will ever be produced with serum is negligible.
Yes, as I've said for many years, the use of fetal bovine serum (FBS) is not a real bottleneck for the industry. Expect other products to follow. The amount of CM that will ever be produced with serum is negligible.
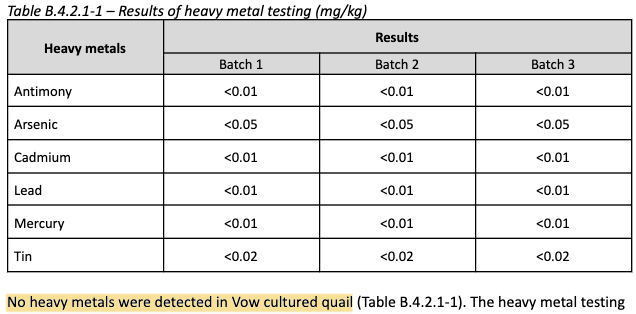
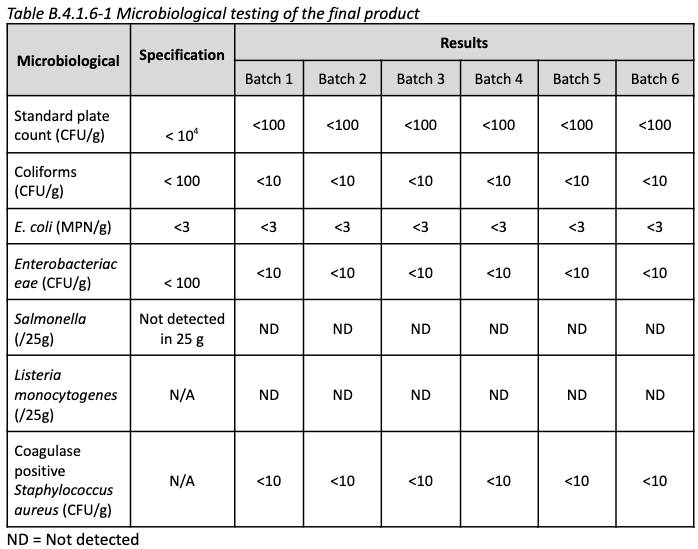
Safety: The cells are tested for pathogens prior to banking, as well as common foodborne contaminants such as E. coli and Salmonella following cell culture
All results are negative. Another advantage of CM is quality control of the cells, ensuring their safety/sterility.
All results are negative. Another advantage of CM is quality control of the cells, ensuring their safety/sterility.
Product: The cultivated quail cells are intended to be used as an ingredient in meat products, where they may be mixed with other food ingredients such as plant proteins or fats (making it hybrid/blended).
The quail cells are intended to make up the bulk of the product.
The quail cells are intended to make up the bulk of the product.
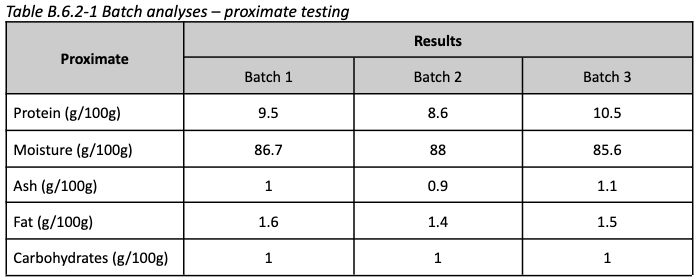
Nutrition: The product's macronutritional profile suggests its water content is higher than conventional quail, its protein content is roughly half, and it contains very little fat
This may be the result of using fibroblasts rather than full tissue (containing muscle, fat, etc)


This may be the result of using fibroblasts rather than full tissue (containing muscle, fat, etc)
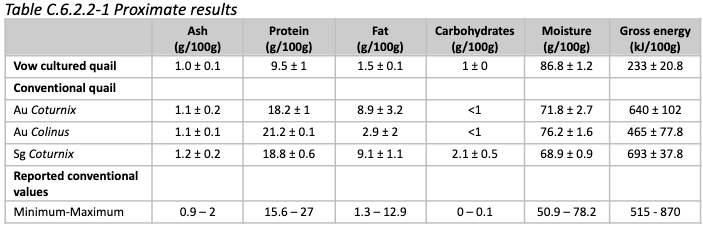
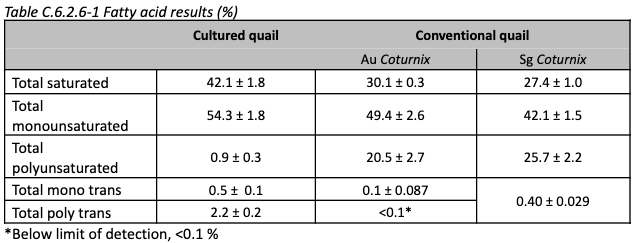
Nutrition: Amino acid and fatty acid results are shown below, compared to conventional quail. No cholesterol values are provided.
There is some variation noted


There is some variation noted
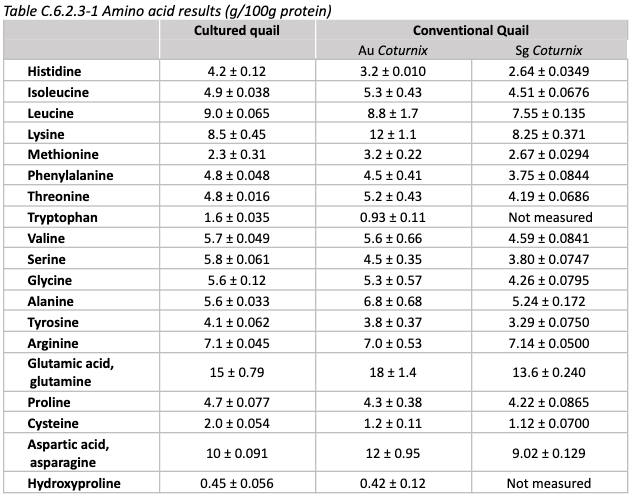
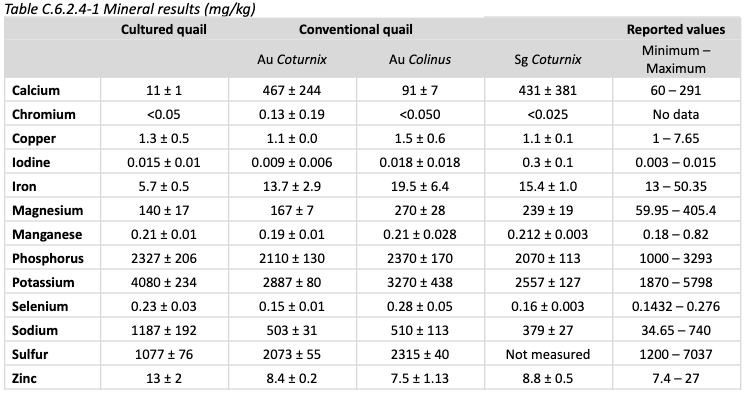
Nutrition: Mineral content is shown below, compared to conventional quail
Minerals in comparatively low amounts include calcium, iron, and sulfur. Minerals in high amounts include potassium, sodium, and zinc.
None of these are expected to result in safety issues.
Minerals in comparatively low amounts include calcium, iron, and sulfur. Minerals in high amounts include potassium, sodium, and zinc.
None of these are expected to result in safety issues.
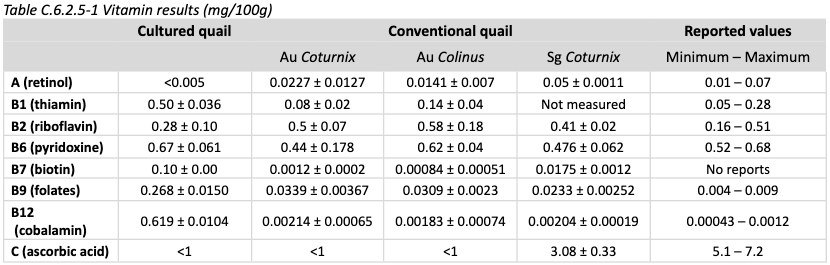
Nutrition: Vitamin content is shown below, compared to conventional quail.
Vitamins in comparatively high amounts include B1, B7, B9, and B12.
B7, B9, and B12 are all quite high, although this is not anticipated to result in any safety issues.
Vitamins in comparatively high amounts include B1, B7, B9, and B12.
B7, B9, and B12 are all quite high, although this is not anticipated to result in any safety issues.
Labeling: FSANZ discusses labeling of the product. Due to how the law is written, the product can't be called meat because the cells are derived from an embryo.
The regulator suggests using the term "cell-cultured" for food ID purposes, based on a consumer study they conducted.
The regulator suggests using the term "cell-cultured" for food ID purposes, based on a consumer study they conducted.
The dossier was originally submitted on Jan 20th, 2023, so it took roughly 11 months for it to be evaluated up until this point. The product is also under review in Singapore.
The next steps are two public commentary periods, which will inform the final approval process in 2024
The next steps are two public commentary periods, which will inform the final approval process in 2024
Website containing the safety submission, accompanying documentation, and assessment/summary from the regulators:
foodstandards.gov.au/food-standards…
foodstandards.gov.au/food-standards…
There are 2 products currently approved for sale in the United States.
See this thread for the FDA documentation of the approved cultivated chicken produced by @UPSIDEfoods
See this thread for the FDA documentation of the approved cultivated chicken produced by @UPSIDEfoods
https://x.com/elliotswartz/status/1597231272681566208?s=20
See this thread for the FDA documentation of the approved cultivated chicken produced by @GOODMeat
https://x.com/elliotswartz/status/1651566348986900481?s=20
@threadreaderapp unroll
• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter