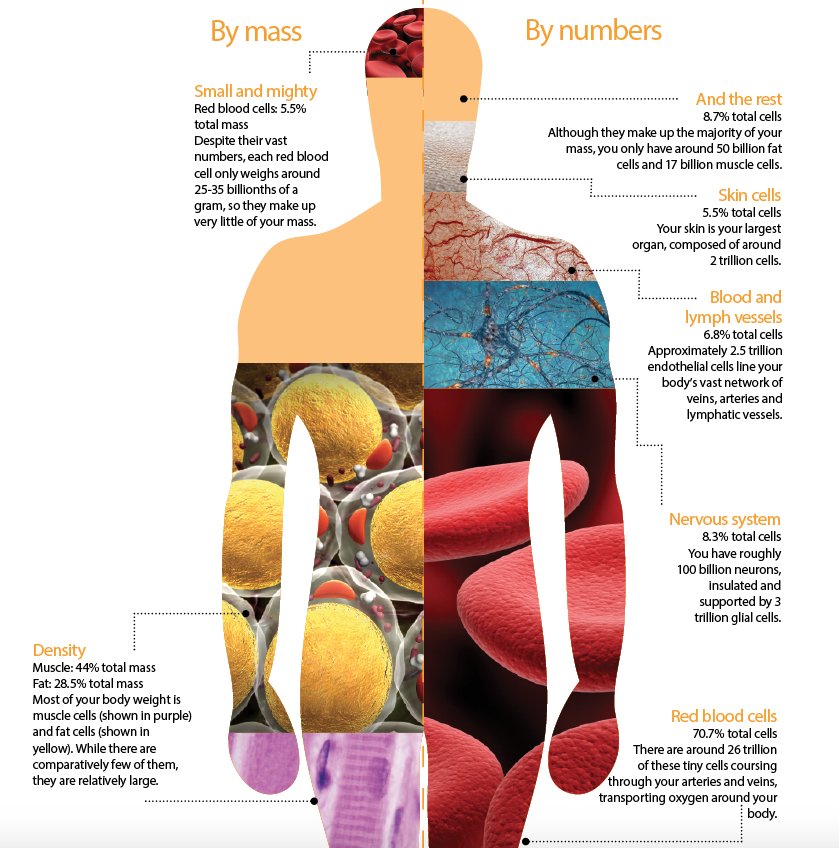
2) "Organ Involvement in COVID-19: A Molecular Investigation of Autopsied Patients"
mdpi.com/2076-2607/10/7…

mdpi.com/2076-2607/10/7…

4) "Molecular Structure, Pathophysiology, and Diagnosis of COVID-19"
researchgate.net/publication/34…

researchgate.net/publication/34…

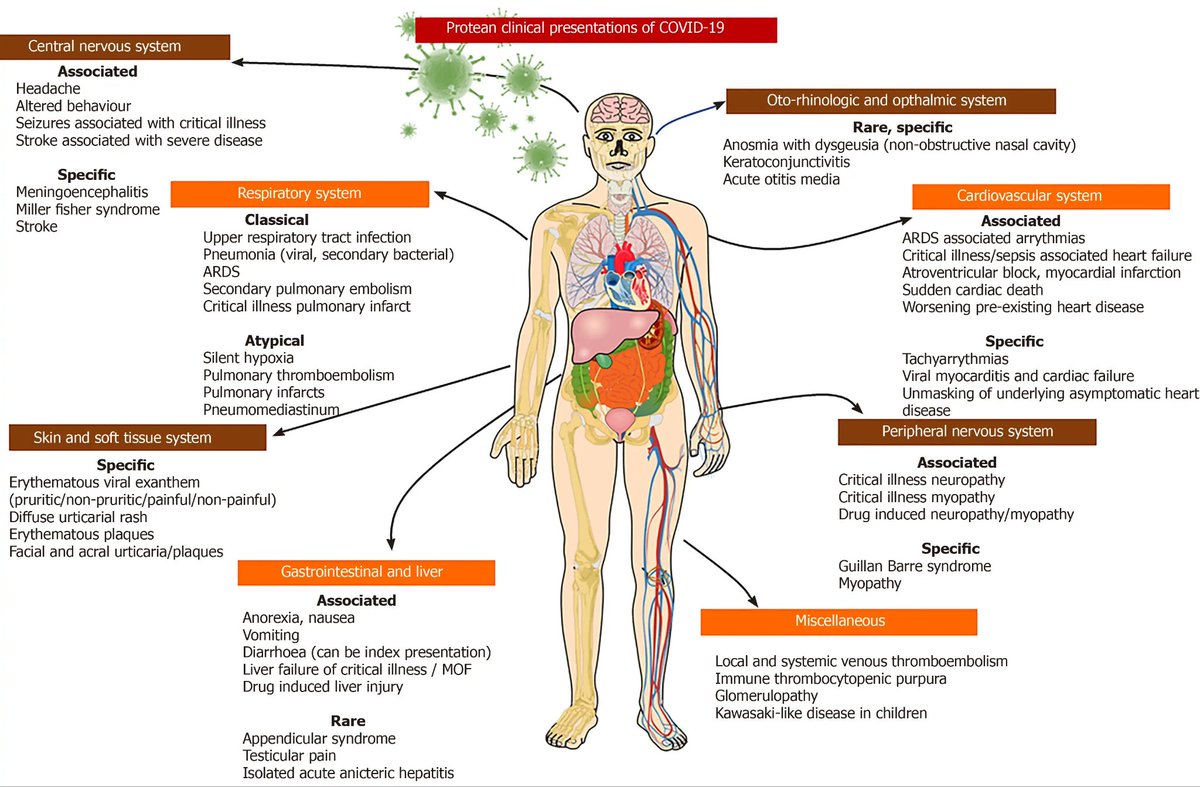
6) "One disease, many faces-typical and atypical presentations of SARS-CoV-2 infection-related COVID-19 disease"
wjgnet.com/2307-8960/full…

wjgnet.com/2307-8960/full…
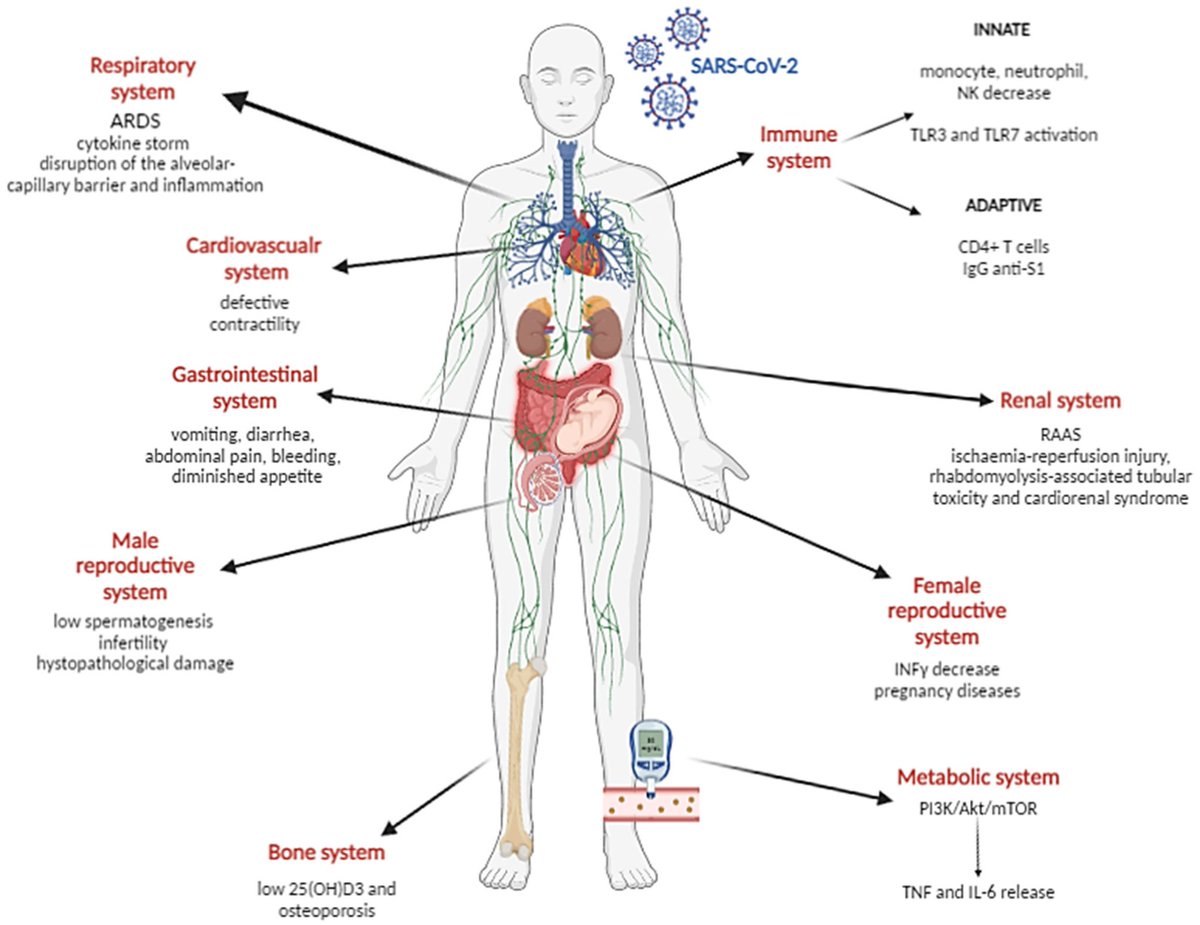
7) "Systemic and organ-specific immune-related manifestations of COVID-19"
nature.com/articles/s4158…

nature.com/articles/s4158…

8) "From Cell to Symptoms: The Role of SARS-CoV-2 Cytopathic Effects in the Pathogenesis of COVID-19 and Long COVID"
mdpi.com/1422-0067/24/9…

mdpi.com/1422-0067/24/9…

• • •
Missing some Tweet in this thread? You can try to
force a refresh