No, #LongCovid (PASC) is real!
Now, a new, cellular level study has also proved that!
The study finds that altered mitochondrial respiration in peripheral blood mononuclear cells of LongCovid patients may be contributing to the etiology of PASC. 1/
Now, a new, cellular level study has also proved that!
The study finds that altered mitochondrial respiration in peripheral blood mononuclear cells of LongCovid patients may be contributing to the etiology of PASC. 1/
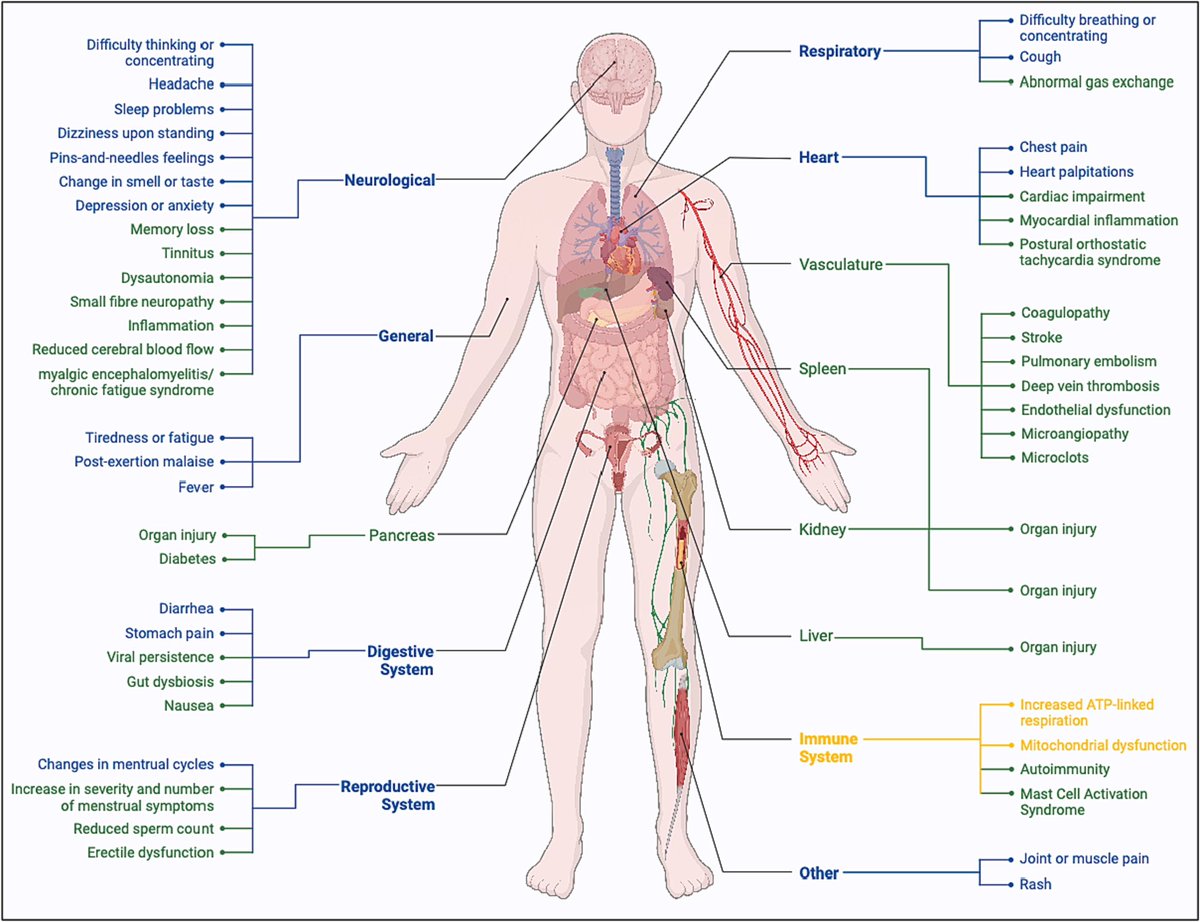
The pathomechanisms and cellular aspects of #LongCovid (PASC) are also not entirely understood. PASC has been theorized to result from damaged tissue, inflammation, thrombosis, or autoreactive B and T cells. 2/
Additionally, reservoirs of SARS-2 in tissues & immune dysregulation have been proposed as causes
One particular concern is the role of mitochondrial function or dysfunction in LongCovid (PASC). Mitochondrial dysfunction has been described in acute severe SARS2 infection. 3/
One particular concern is the role of mitochondrial function or dysfunction in LongCovid (PASC). Mitochondrial dysfunction has been described in acute severe SARS2 infection. 3/

It remains unclear whether the disturbances in mitochondria identified in participants during acute COVID-19 infection are also present in PASC. 4/
In a new study, researchers measured peripheral blood mononuclear cells (PBMC) mitochondrial oxygen consumption in participants w/out COVID (COVID-), participants w/ a history of COVID & full recovery (COVID + No PASC) and participants w/ PASC symptoms (COVID + PASC +) 5/ 

Compared to COVID + no PASC, the proportion of respiration linked to ATP-linked respiration was significantly higher among PASC + 6/
Increases in basal respiration, ATP-linked respiration, maximal respiration, spare respiratory capacity, and non-mitochondrial respiration were associated with increases in the likelihood of PASC. 7/ 

These findings suggest that the energetic needs of PASC PBMC are greater than COVID + no PASC and COVID- PBMC. 8/
Data conclude that mean basal respiration, ATP-linked respiration, maximal respiration, spare respiratory capacity, proton leak, non-mitochondrial respiration are highest in PASC PBMC. 9/ 

These findings suggest the hypothesis of an increase in the relative number or activity of circulating monocytes in PASC, as monocytes have the greatest number of mitochondria and reprogram their metabolism in response to stimulation to support inflammatory responses. 10/
PBMC exposure to SARS-CoV-2 has been shown to result in the detection of viral RNA, particularly in monocytes, leading to a monocyte-accentuated, JAK/STAT-dependent, innate-immune response. 11/ 

Furthermore, SARS-CoV-2 antigens, notably full-length Spike protein, circulate in the plasma of PASC participants to 12 months after infection. 12/
These circulating antigens could stimulate monocytes to produce an omnipresent state of inflammation, requiring increased ATP-linked respiration and leading to the symptoms of PASC. 13/
The findings that mitochondria in PASC PBMC exhibit increased oxygen consumption could explain the discoveries of lactic acidosis and impaired fatty acid oxidation in PASC persons during physical activity. 14/
As the elevated oxygen consumption in the blood would reduce the amount of oxygen available for muscles. This anaerobic environment would increase muscular glycolysis, produce more lactic acid, and impair mitochondrial-dependent lipid catabolism. 15/
The findings suggest that cellular bioenergetics are altered in PASC with increased oxygen consumption and proportion of respiration linked to ATP-linked respiration, which may reflect a crucial pathogenic mechanism associated with ongoing inflammation. 16/
Further, these findings suggest that the increase in the energetic needs of PASC PBMC contributes to the symptoms of PASC. 17/
The cells in the bone marrow known as human hematopoietic stem/progenitor cells (HSC) highly express ACE2 and TMPRSS2, making them susceptible to SARS-CoV-2 during the initial infection. 18/ 

This susceptibility is demonstrated in research showing HSC will undergo pyroptosis if exposed to SARS-CoV-2 and hyperactivated. Additionally, SARS-CoV-2 RNA has been detected in the bone marrow of post-mortem samples. 19/
PBMC lack ACE2 receptor expression and do not support productive SARS-CoV-2 infection but generate a monocyte-accentuated immune response upon stimulation with SARS-CoV-2. 20/
The results herein lead to two novel hypotheses to describe the pathomechanisms leading to PASC, one involving immune dysregulation facilitated by monocytes and the other involving a residual viral reservoir in human hematopoietic stem cells (HSC) 21/
These findings suggest that cellular bioenergetics are altered in PASC with increased oxygen consumption and proportion of respiration linked to ATP-linked respiration. 22/
This may reflect a crucial pathogenic mechanism associated with ongoing inflammation. Further, these findings suggest that the increase in the energetic needs of PASC PBMC contributes to the symptoms of PASC. 23/
The results herein lead to two novel hypotheses to describe the pathomechanisms leading to PASC, one involving immune dysregulation facilitated by monocytes and the other involving a residual viral reservoir in HSC. 24/
sciencedirect.com/science/articl…
sciencedirect.com/science/articl…
• • •
Missing some Tweet in this thread? You can try to
force a refresh
























