Delighted to share our latest work on #longCOVID - sex differences in symptoms and immune signatures. Led by @SilvaJ_C @taka_takehiro @wood_jamie_1 et al. with @LeyingGuan & @PutrinoLab. We find a striking inverse correlation btw testosterone levels and symptom burden👇🏼 (1/)
medrxiv.org/content/10.110…
medrxiv.org/content/10.110…
This work leverages data from our recent Mount Sinai-Yale long COVID "MY-LC" study with the @PutrinoLab. This time, we asked the question, "Are there differences in symptoms and immune signatures of ♀️ vs. ♂️ with LC"? (2/)
nature.com/articles/s4158…

nature.com/articles/s4158…

While some symptoms were equally frequent in females and males, many were more frequent in females (e.g., swelling, headaches, muscle pain, cramps) than males. The top distinguishing symptoms of LC status by sex were hair loss in females and sexual dysfunction in males. (3/) 

What organ-specific symptoms correlate with one another in females vs. males? In females (left), temperature-related symptoms and musculoskeletal symptoms were strongly associated with other symptoms. In males (right), ear nose and throat (ENT) symptoms were most associated with other symptoms. (4/)

What about immune signatures? Females with LC had increases in exhausted and cytokine-secreting T cells and antibodies against lytic antigens from herpesviruses, suggestive of recent reactivation. (5/) 

In contrast, males with LC had elevated NK cells, reduced monocytes and plasmacytoid dendritic cells. They also had increases in TGF-beta, APRIL and IL-8, features not significantly different in females with LC. (6/) 

Females with LC had significantly lower testosterone levels. Using logistic regression, @SilvaJ_C found testosterone to be the top negative hormone predictor of LC status in females based on per unit odds ratio. Thus, in females, the lower the testosterone, the more likely to have LC. (7/)

In males, those with LC had lower levels of estradiol, and estradiol was found to be the top negative predictor hormone of LC status. (8/) 

Next, @silvaJ_C took a different machine-learning approach. This time he divided the MY-LC cohort into training vs. testing sets. He took cellular profiles and plasma factors but excluded hormones within females and males separately and calculated sex-specific immune scores. (9/) 

This strategy successfully predicted LC status in females (86% accuracy) and males (87% accuracy). Meaning that cellular and soluble immune features alone can distinguish those with LC in both sexes pretty accurately. (10/) 

Using this modeling strategy, we found that female LC immune signatures were associated with higher symptom burden, organ involvement and dysautonomia in males with LC. Conversely, male LC immune signatures correlated with lower symptom burden, dysautonomia and neurocognitive symptoms in females with LC! Overall, male LC immune signatures meant less symptoms in LC. (11/)

So what is driving the male LC immune signatures? Testosterone! Regardless of sex, those with higher testosterone levels had higher male LC immune signatures. Those with lower testosterone had higher female LC immune signatures. (12/) 
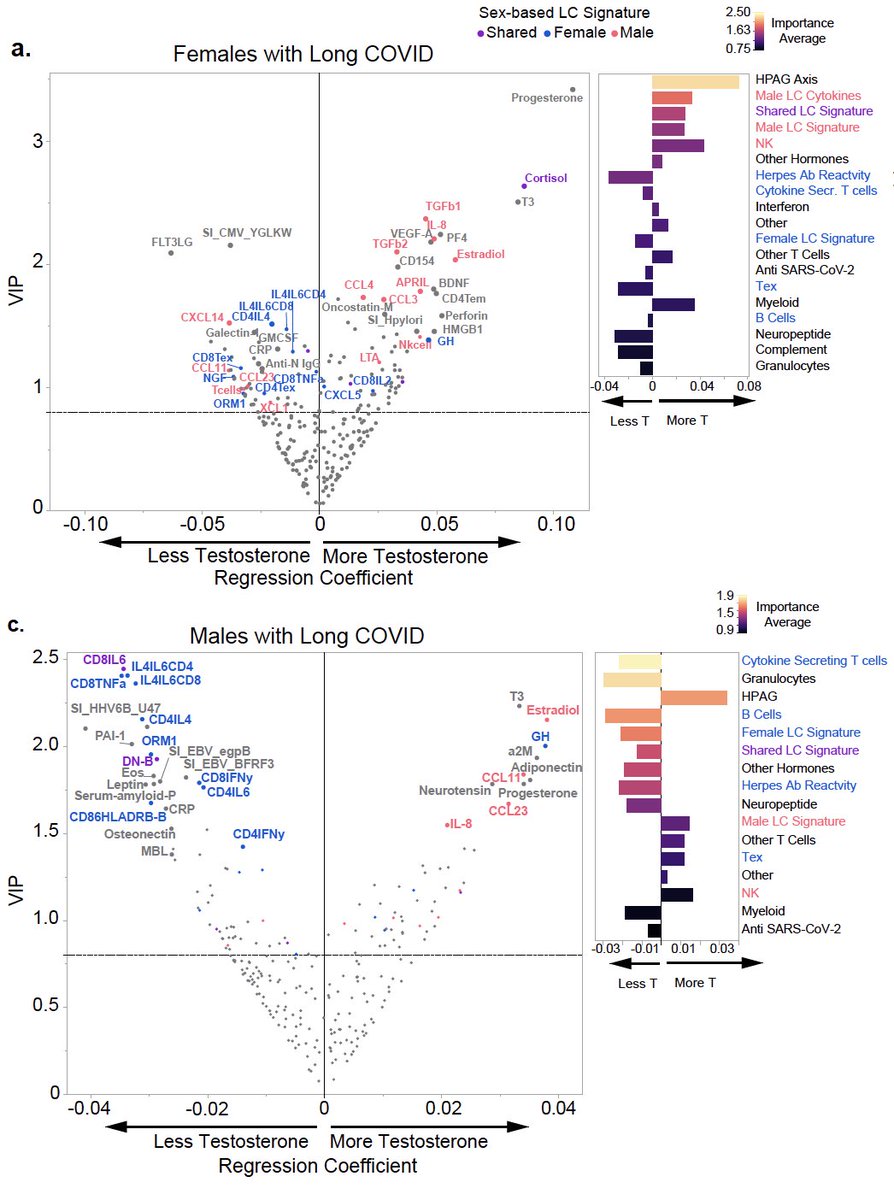
Finally, testosterone levels could significantly predict not just LC status but also symptom burden and organ system involvement in individuals with LC irrespective of sex. (13/) 

To summarize, we find significant differences in symptoms and immune signatures in females vs. males with long COVID. (14/) 

There is a fascinating backstory to this study I wish to share (with permission). In August of 2022, @tarazupancic DM me about a profound improvement in the long COVID symptoms of her child after receiving testosterone for gender-affirming care. I discussed this at our weekly long COVID lab science meeting. This is how it all started. So grateful to Tara for sharing this key insight with us 🙏🏼🙏🏼 (15/)
As always, this was a huge undertaking by many dedicated scientists and clinicians, and the amazing contributions of patients who donated their time, effort and blood samples. Incredible teamwork with the Mount Sinai team @PutrinoLab. Kudos to @SilvaJ_C, @taka_takehiro, @wood_jamie_1, @peowenlu, @S_Tabachnikova, @JeffGehlhausen, @KerrieGreene_ , @bornali_27, Valter Silva Monteiro, @carolilucas, @rahuldhodapkar, @marioph13, Kathy Kamath, @tianyangmao, Dayna Mccarthy, @RMedzhitov, @david_van_dijk, @hmkyale and @LeyingGuan!!

• • •
Missing some Tweet in this thread? You can try to
force a refresh