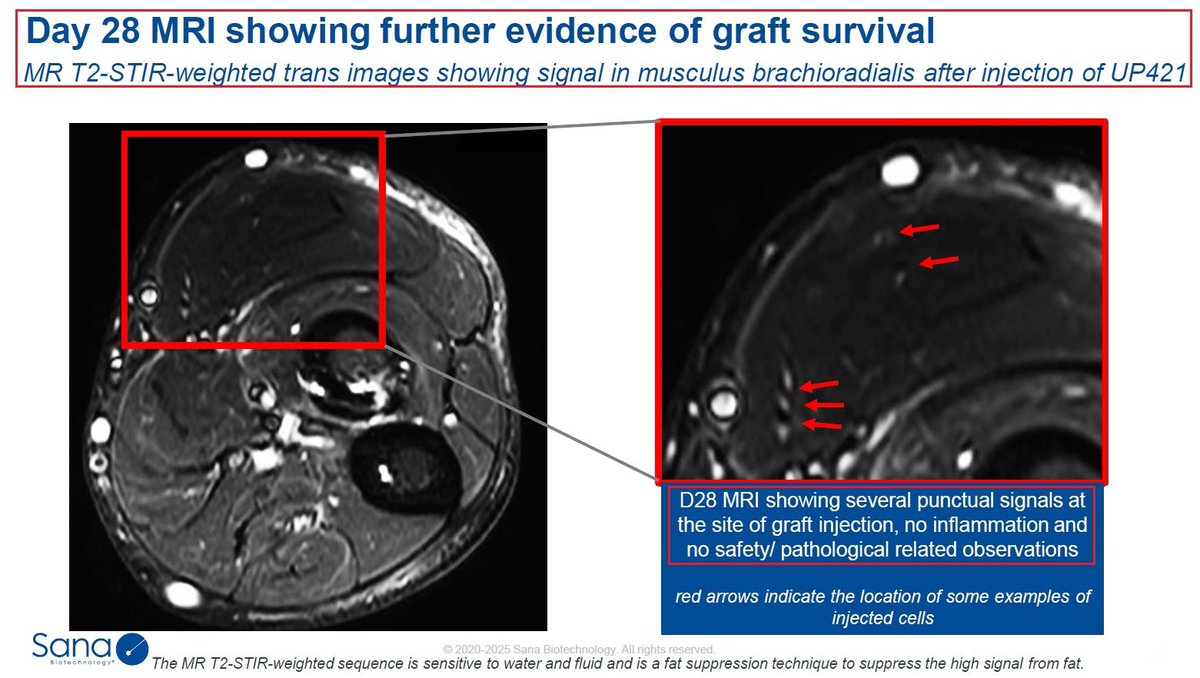
1/As promised & after reading $NTLA latest Q4 & full year 2023 financial report here is my impression regarding @intelliatx latest corporate status. As always I have focused only on the main issues that I found to be the most interesting & relevant. Here is my 🧵👇 $XBI #BioTech 

2/IMO the most significant corporate event was @intelliatx collaboration signed with @ReCodeTx - which uses tissue-specific delivery to power mRNA & gene correction therapeutics to develop novel medicines for the treatment of Cystic fibrosis based on $NTLA Gene Editing platform 
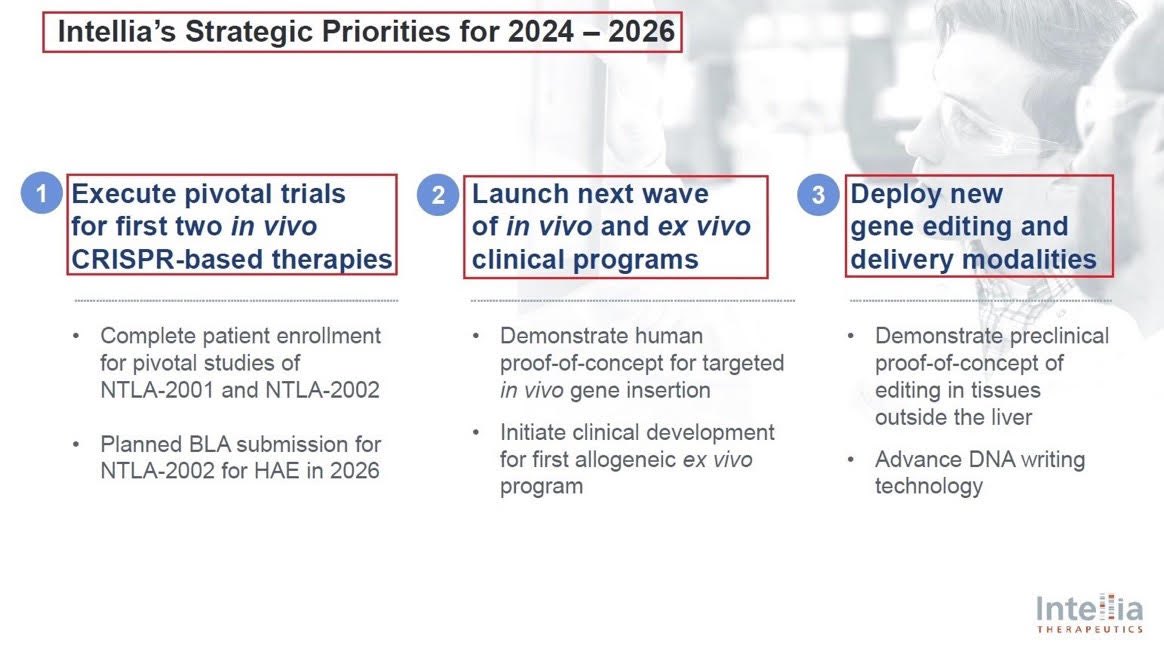
3/Another major development - which was recently presented in the @jpmorgan’s health conference, was @intelliatx’s restructured pipeline & its new 2024-2026 main Strategic Priorities:
1. Trials for its 2 in vivo #CRISPR platforms - $NTLA-2001 & 2002
2. POC for $NTLA new CRISPR-in vivo targeted gene insertion & allogeneic ex vivo program
3. Developing new Gene editing programs outside the liver
4. DNA writing
1. Trials for its 2 in vivo #CRISPR platforms - $NTLA-2001 & 2002
2. POC for $NTLA new CRISPR-in vivo targeted gene insertion & allogeneic ex vivo program
3. Developing new Gene editing programs outside the liver
4. DNA writing
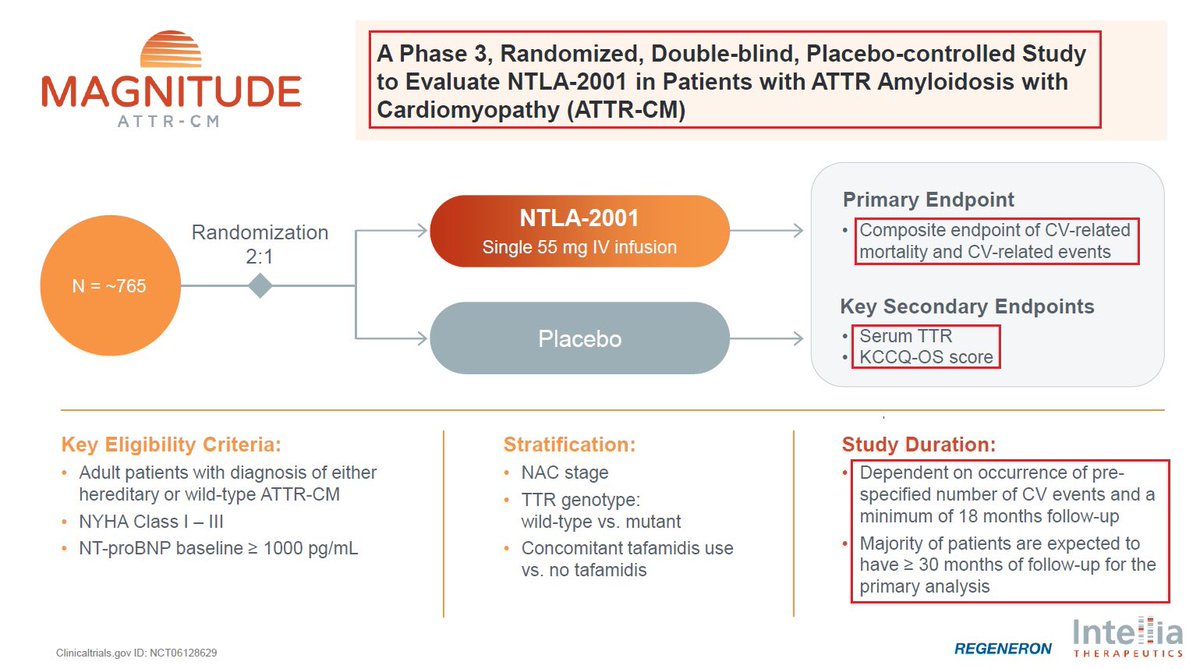
@jpmorgan @intelliatx 4/Following the restructuring of its clinical portfolio & focusing on its main Gene editing programs - $NTLA - 2001, 2002 & 3001 - @intelliatx anticipates to reach the following key clinical milestones during 2024👇 

5/NTLA-2001 is @intelliatx’s In-Vivo CRISPR Cas9 Gene editing program which is aimed to treat Transthyretin Amyloidosis-ATTR. $NTLA-2001 could potentially halt and reverse the disease by knocking out the TTR gene with a single dose-creating a “one & done” treatment for patients 

@intelliatx 6/@intelliatx is actively enrolling patients, including in the U.S., in the pivotal Phase 3 MAGNITUDE trial. $NTLA is on track to dose the first patient in Q1 2024, continues to open new clinical sites & plans to present updated data from the ongoing Phase 1 study in 2024. 
@intelliatx 7/@intelliatx’s second program is $NTLA-2002 which aims to knock out the KLKB1 gene in the liver thus reducing total plasma kallikrein protein & activity - a key mediator of HAE hereditary angioedema which affects more than 15,000 patients globally & has a $4B+ market size 
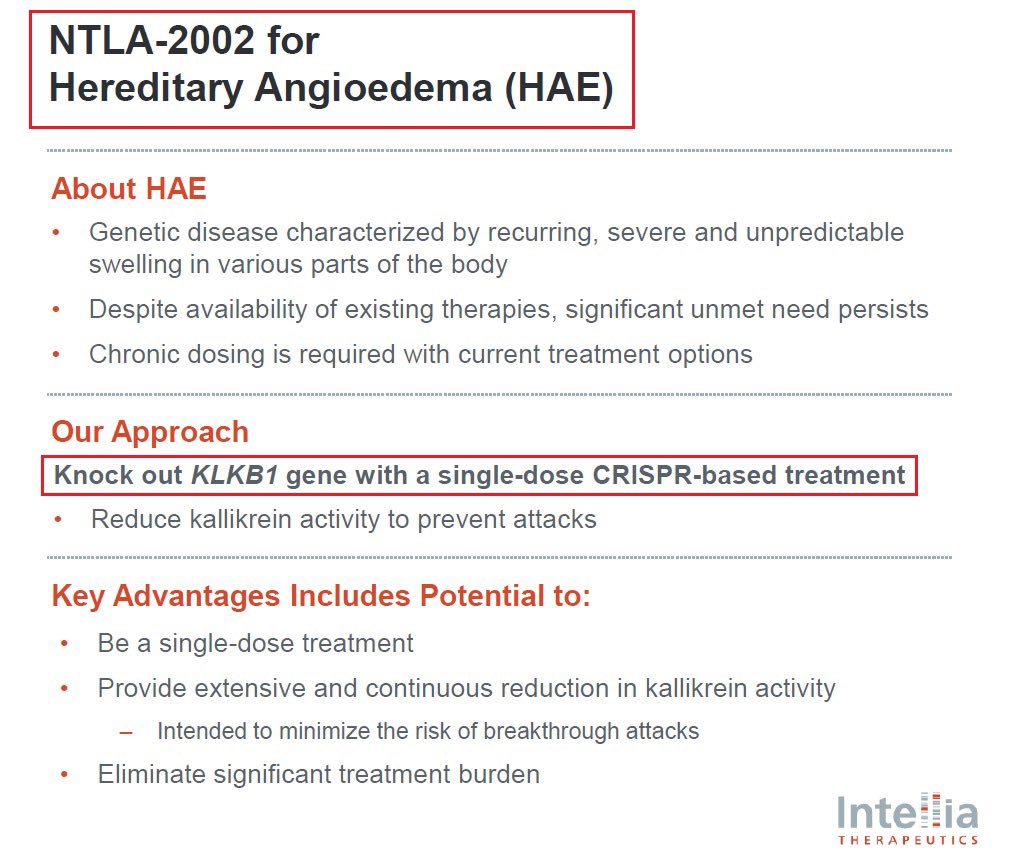
@intelliatx 8/@intelliatx has announced in January that it had completed the enrollment and dosing in the Phase 2 portion of the Phase 1/2 study in adults with HAE. $NTLA plans to present updated data from the Phase 1 and new data from the Phase 2 portion during 2024. 
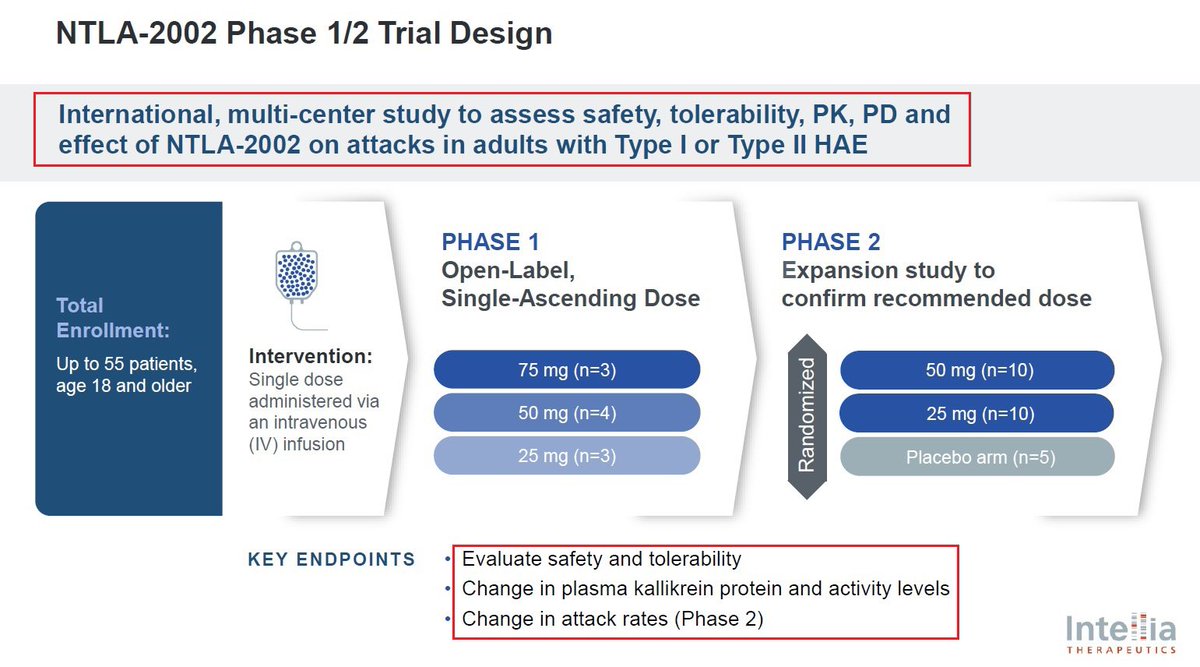
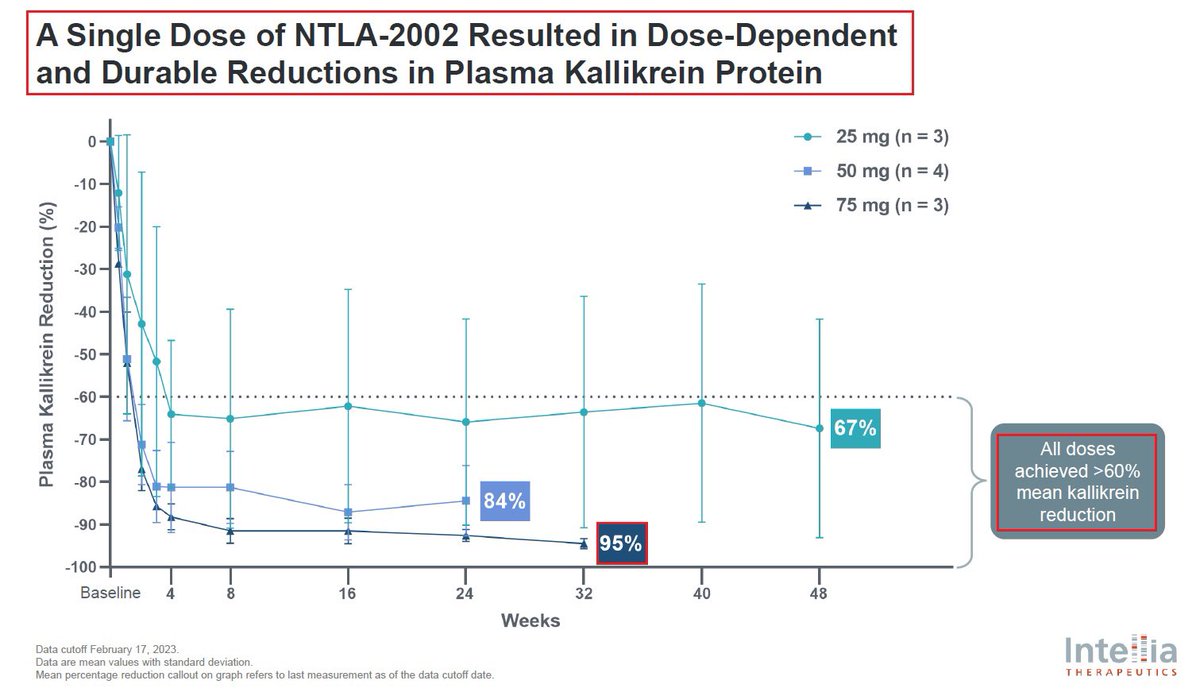
9/In January @intelliatx reported preliminary positive results from its Phase 1 portion of the Phase 1/2 study of $NTLA-2002 which was published in the @NEJM. The reported data showed that a single dose of NTLA-2002 led to a 95% mean reduction in monthly HAE attack rate across all 10 patients in the Phase 1 portion & was well tolerated at all dose levels. The most frequent adverse events reported were mild, transient infusion-related reactions and fatigue.
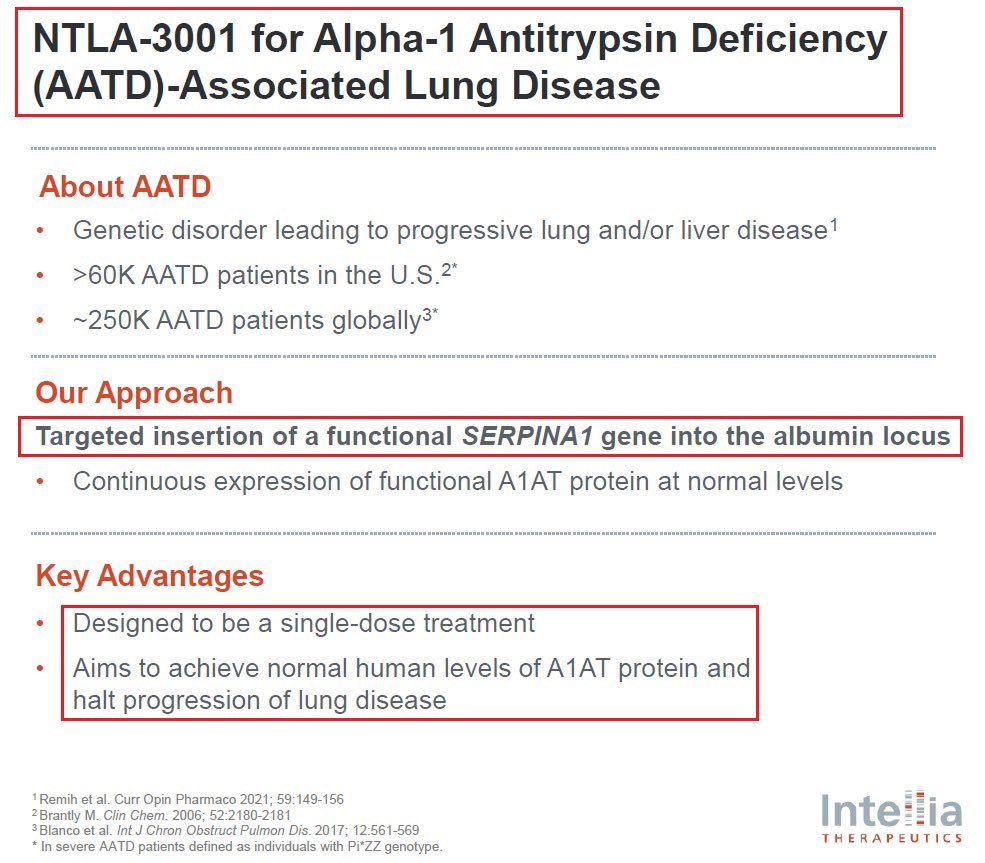
10/@intelliatx $NTLA-3001 is a wholly owned, first-in-class CRISPR-mediated in vivo targeted gene insertion development candidate for the treatment of AATD-associated lung disease. It is designed to precisely insert a healthy copy of the SERPINA1 gene, which encodes the alpha-1 antitrypsin (A1AT) protein, with the potential to restore permanent expression of functional A1AT protein to therapeutic levels after a single dose. In December 2023, Intellia submitted a Clinical Trial Application (CTA) to initiate a first-in-human, Phase 1 study of NTLA-3001. The Company plans to dose the first patient in 2024.
@intelliatx @NEJM 11/One of @intelliatx’s major strengths is its multiple collaborations: 1)the acquisition of Rewrite therapeutics 2)@OnkTherapeutics - development of CRISPR-edited NK Cell therapy for Cancer 3)@KyvernaT - development of KYV-201 - a CD19 CAR-T cell for autoimmune diseases. 
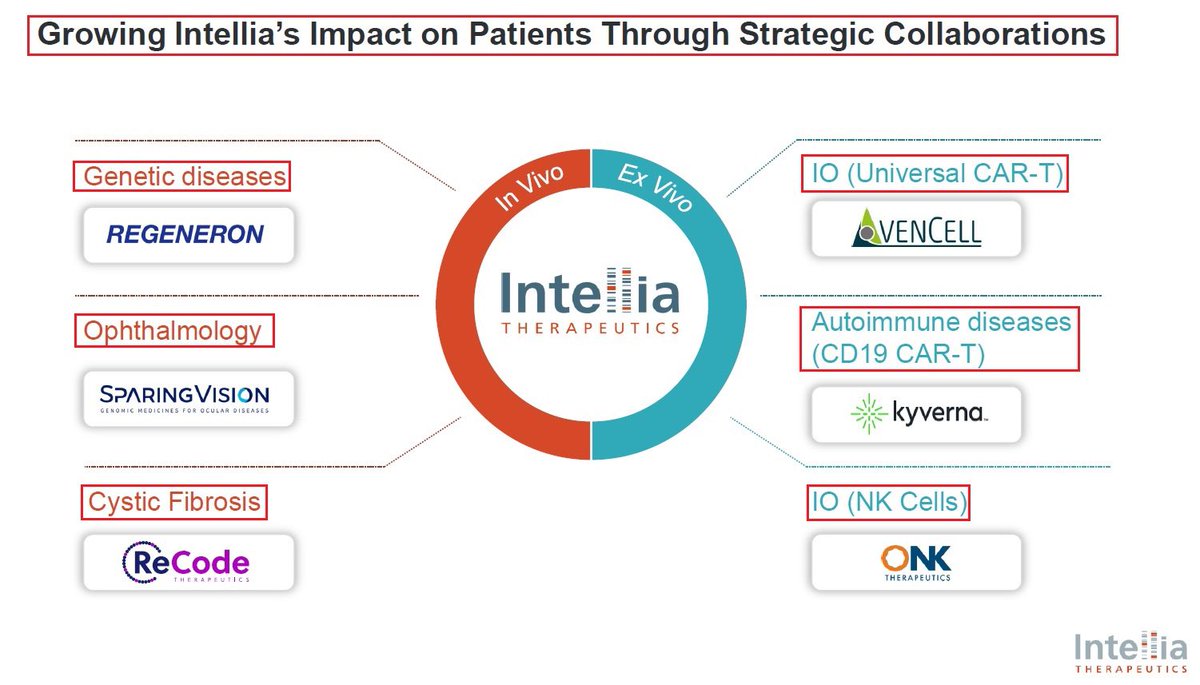
12/@intelliatx most important collaboration is the one with @Regeneron. In October 2023, $NTLA & $RGNX announced an expanded research collaboration to develop additional in vivo CRISPR-based gene editing therapies focused on neurological & muscular diseases. In addition, Regeneron also exercised its option to extend the existing technology collaboration term with Intellia for two more years. The technology collaboration term now extends to April 2026, and Intellia will receive a $30M payment due in April 2024.

13/Another important $NTLA collaboration is the one signed with @SparingVision - a French biotech company focused on developing treatments for ocular Genetic Diseases. SPVN will utilise @intelliatx CRISPR Cas9 platform & will develop new treatments for up to 3 selected targets👇
https://twitter.com/yaireinhorn/status/1448323985771483136
@SparingVision @intelliatx 14/In 2021 @intelliatx had acquired 10% of @SparingVision’s equity in exchange for up to $200M in future funding around milestones & royalties. @SparingVision will select 3 drug candidates & $NTLA will own 2 of the 3, owning all commercial rights in the 🇺🇸 + 50% of all non US 

@SparingVision @intelliatx 15/In September @SparingVision & $NTLA announced that they have selected an undisclosed 2ND target as part of their strategic collaboration to develop novel genomic medicines utilizing @intelliatx CRISPR-based Gene Editing technologies for the treatment of ocular diseases. $XBI 

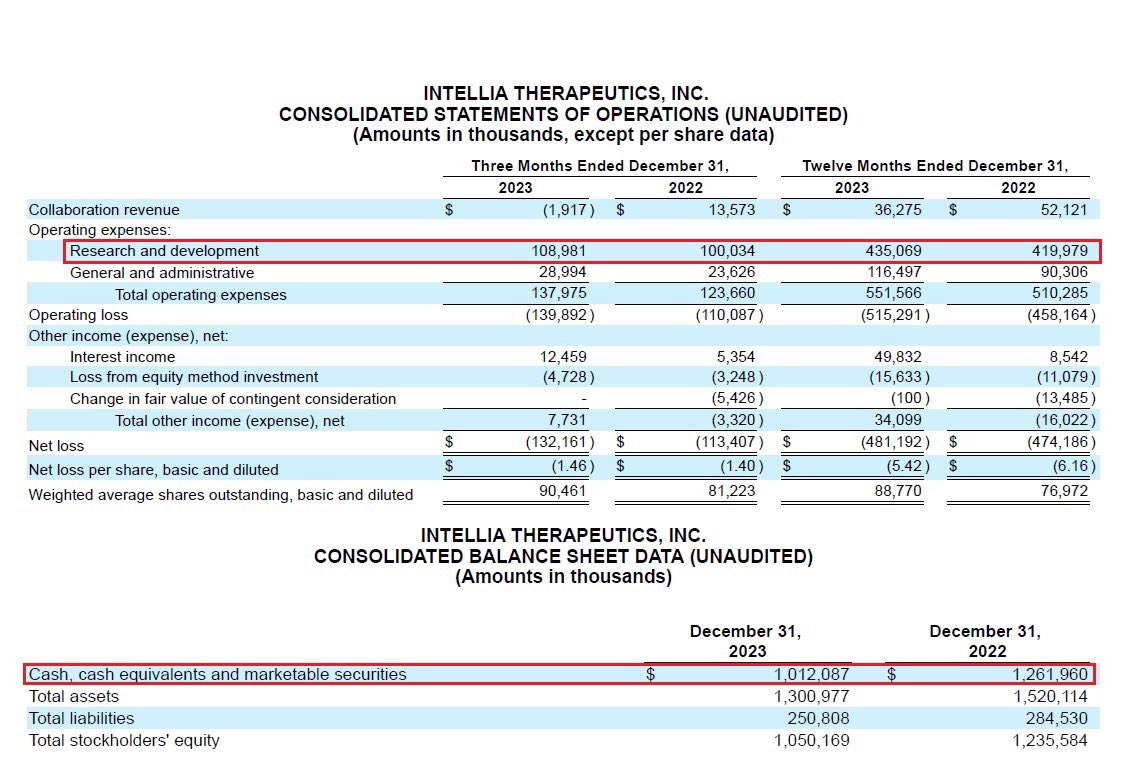
@SparingVision @intelliatx 16/As of 12/31/23 $NTLA had capital resources of $1B & quarterly R&D expenses of $109M. IMO despite the high cash burn, @intelliatx’s current cash position of ~ $1B will enable it to continue in developing its pipeline & to initiate additional acquisitions and collaborations. 
@SparingVision @intelliatx 17/@intelliatx remains very promising due to its broad collaboration agreements alongside a diverse pipeline. After receiving an IND clearance for $NTLA-2001 thus clearing the way to the US market & advancing $NTLA-2002 into Phase 3 - ‘24 is expected to be a strong year for $NTLA 

Please feel free to share, retweet or Bookmark this🧵so that those on @X - who are interested in Gene editing, CRISPR, BioTech and Genomics will be able to access this resource and as always I would be more than happy to read your thoughts. $XBI $NTLA
https://twitter.com/yaireinhorn/status/1764642249470591397
• • •
Missing some Tweet in this thread? You can try to
force a refresh