
Science and Innovation. Special interest in BioTech, Pharma, Gene Editing and CRISPR 🧬. Sharing my thoughts & 📊 on the Global Economy & Financial Markets. 📈
2 subscribers
How to get URL link on X (Twitter) App

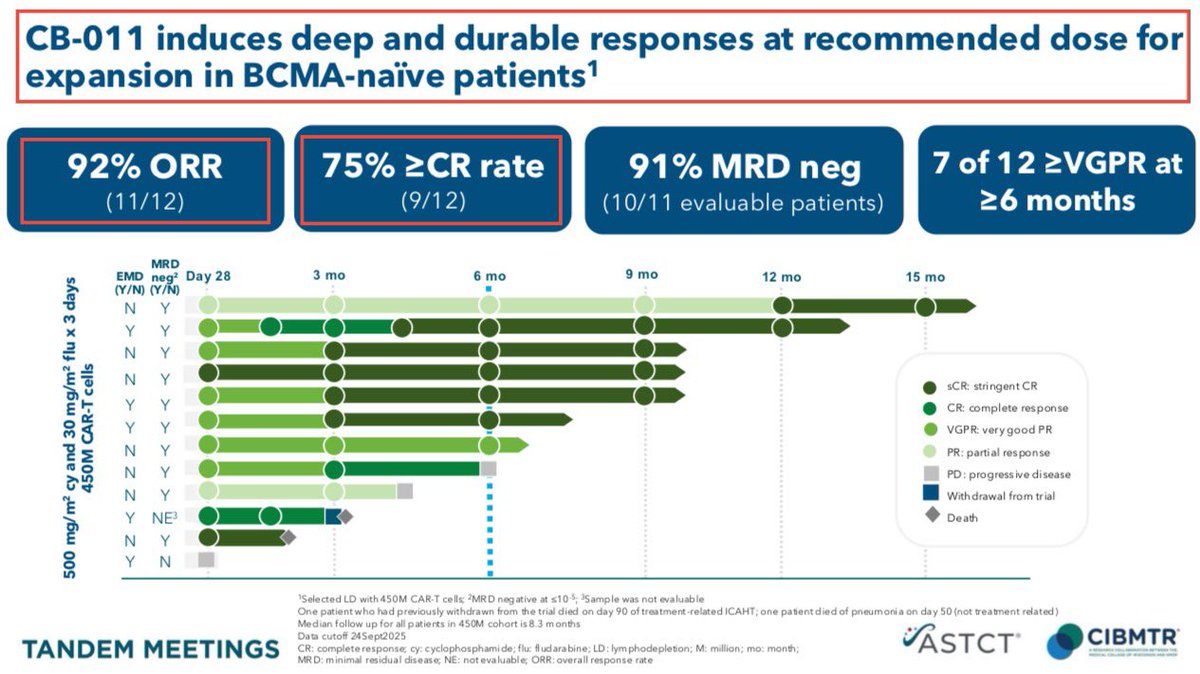
 2/Multiple myeloma is a type of blood cancer that develops in the patient’s bone marrow when plasma cells become cancerous and produce abnormal proteins instead of functional antibodies. Common symptoms include persistent bone pain (often back or ribs), fatigue, frequent infections, kidney damage and high calcium levels. While modern treatments (chemotherapy, targeted therapy, stem cell transplants) can manage the disease for years - Multiple myeloma is still considered incurable and present a significant unmet medical need.
2/Multiple myeloma is a type of blood cancer that develops in the patient’s bone marrow when plasma cells become cancerous and produce abnormal proteins instead of functional antibodies. Common symptoms include persistent bone pain (often back or ribs), fatigue, frequent infections, kidney damage and high calcium levels. While modern treatments (chemotherapy, targeted therapy, stem cell transplants) can manage the disease for years - Multiple myeloma is still considered incurable and present a significant unmet medical need.
 2/@PrimeMedicine’s proprietary platform - Prime Editing is the only Gene Editing platform which can edit, correct, insert or even delete large DNA sequences in any target tissue. This makes it the most advanced & promising editing technology with $PRME owing its full IP rights.
2/@PrimeMedicine’s proprietary platform - Prime Editing is the only Gene Editing platform which can edit, correct, insert or even delete large DNA sequences in any target tissue. This makes it the most advanced & promising editing technology with $PRME owing its full IP rights. 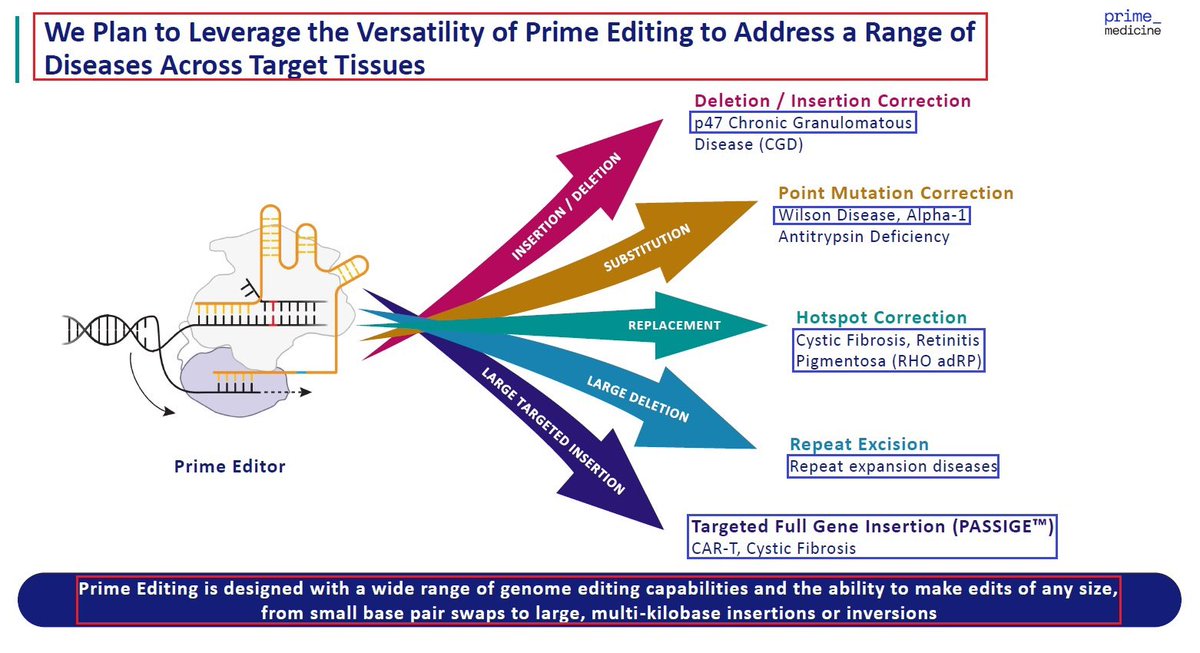

 2/The British government has always considered its life science industry as one of the Crown Jewels of its economy and rightfully so - a 145 billion dollars industry which employs over 300,000 high skilled employees is crucial for the British economy. The importance of the Pharma and Biotech industry has led the British government to a present a new governmental initiative to support the industry and that was introduced by the Labor government just 3 months ago.
2/The British government has always considered its life science industry as one of the Crown Jewels of its economy and rightfully so - a 145 billion dollars industry which employs over 300,000 high skilled employees is crucial for the British economy. The importance of the Pharma and Biotech industry has led the British government to a present a new governmental initiative to support the industry and that was introduced by the Labor government just 3 months ago.

 2/In February of 2022 the U.S. Patent & Trademark Office has issued a crucial decision in favour of the Broad Institute, which validated its patents for CRISPR/Cas9 Gene Editing in human cells. This provided $EDIT with strong IP rights & gave it a huge commercial advantage. $XBI
2/In February of 2022 the U.S. Patent & Trademark Office has issued a crucial decision in favour of the Broad Institute, which validated its patents for CRISPR/Cas9 Gene Editing in human cells. This provided $EDIT with strong IP rights & gave it a huge commercial advantage. $XBI 

 2/Jennifer Doudna is an American biochemist who discovered CRISPR Cas9 as a Gene Editing tool & had received the 2020 Nobel Prize in Chemistry with Emmanuelle Charpentier for their discovery. She also founded several BioTech companies like $NTLA, $CRBU, Scribe, Mammoth & others.
2/Jennifer Doudna is an American biochemist who discovered CRISPR Cas9 as a Gene Editing tool & had received the 2020 Nobel Prize in Chemistry with Emmanuelle Charpentier for their discovery. She also founded several BioTech companies like $NTLA, $CRBU, Scribe, Mammoth & others. 
 2/Type 1 diabetes is a chronic (life-long) autoimmune disease that prevents the patient’s pancreas from making Insulin - an important hormone that regulates the amount of glucose (sugar) in the blood. Type 1 diabetes affects both children and adults & requires daily management with insulin injections and blood sugar monitoring.
2/Type 1 diabetes is a chronic (life-long) autoimmune disease that prevents the patient’s pancreas from making Insulin - an important hormone that regulates the amount of glucose (sugar) in the blood. Type 1 diabetes affects both children and adults & requires daily management with insulin injections and blood sugar monitoring.

 2/All 17 deals made by big Pharma during the first 6 months of ‘24 were $5B or less. During the same period last year, big Pharma made 9 deals, including 2 that were $10B or more. 9 of the 17 deals were for privately held companies, compared to 1 during the same period in 2023.
2/All 17 deals made by big Pharma during the first 6 months of ‘24 were $5B or less. During the same period last year, big Pharma made 9 deals, including 2 that were $10B or more. 9 of the 17 deals were for privately held companies, compared to 1 during the same period in 2023. 

 2/Must of the companies - as you can see in the graph m☝️- use a viral based delivery platform for Gene Therapy / Gene Editing treatments, mostly AAV - Adeno-Associated Virus based delivery. AAV is a naturally occurring virus being transformed into a delivery mechanism by replacing its viral DNA with new DNA, thus making it a precisely coded vector & it is no longer considered a virus, as most of its viral components have been replaced.
2/Must of the companies - as you can see in the graph m☝️- use a viral based delivery platform for Gene Therapy / Gene Editing treatments, mostly AAV - Adeno-Associated Virus based delivery. AAV is a naturally occurring virus being transformed into a delivery mechanism by replacing its viral DNA with new DNA, thus making it a precisely coded vector & it is no longer considered a virus, as most of its viral components have been replaced.
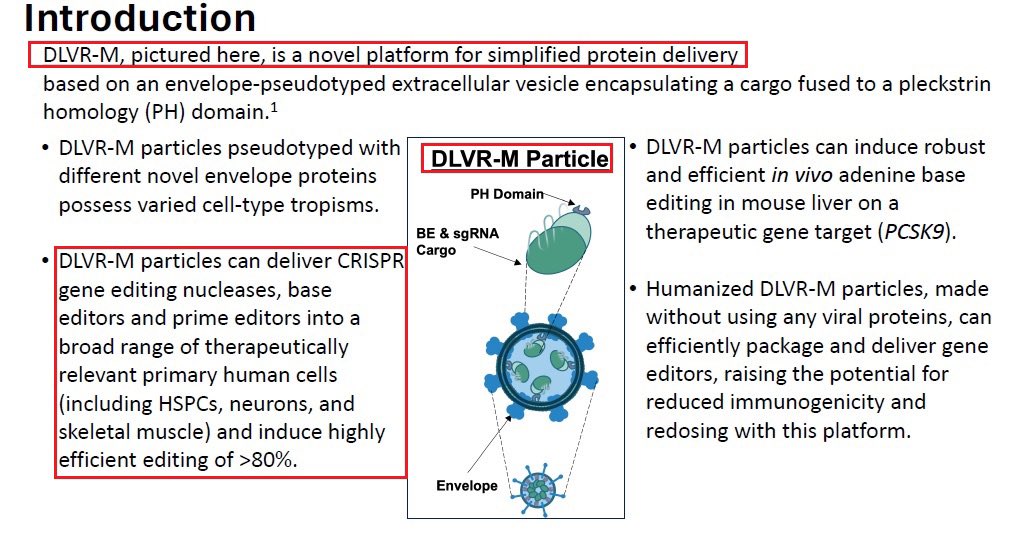
 2/Every Gene Therapy is combined from 2 components - the genetic payload which is the mechanism for fixing the genetic disease or the cure itself & a delivery vehicle which needs to deliver the “package” to a specific human tissue & location. Just Imagine a pickup truck & a box.
2/Every Gene Therapy is combined from 2 components - the genetic payload which is the mechanism for fixing the genetic disease or the cure itself & a delivery vehicle which needs to deliver the “package” to a specific human tissue & location. Just Imagine a pickup truck & a box. 
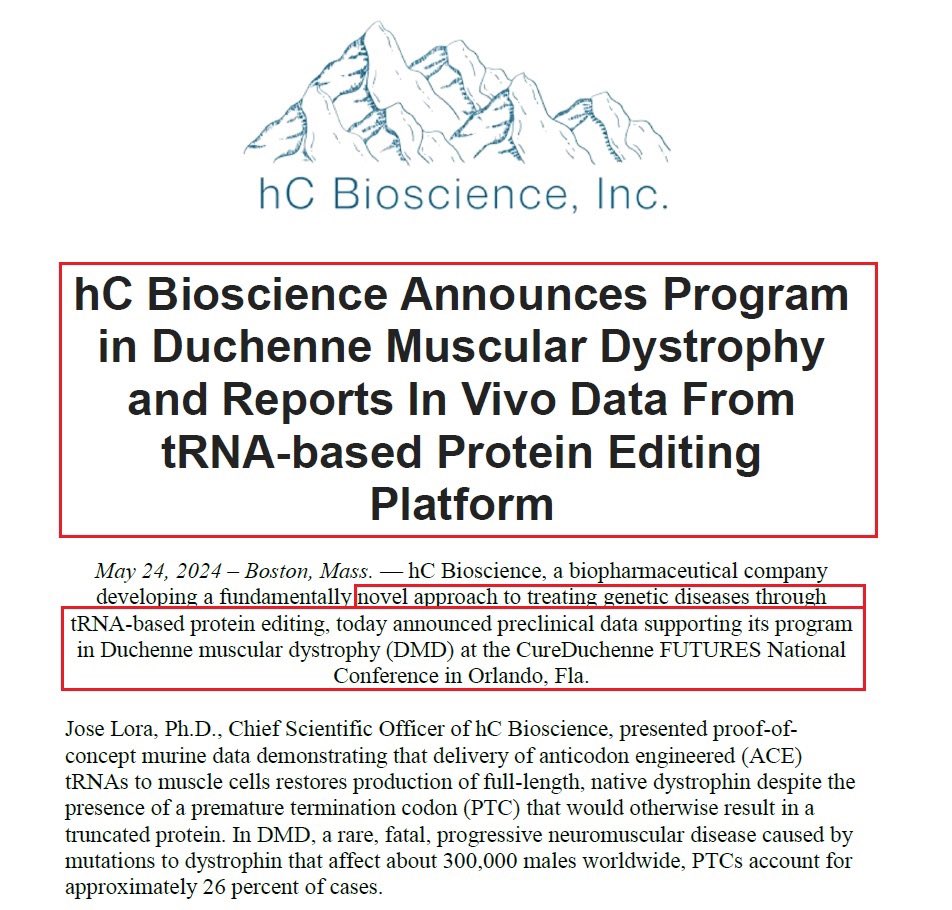
 2/Duchenne is a severe progressive disease which rapidly worsening children’s muscle function often using a wheelchair by early adolescence & eventually needing artificial ventilation to breathe. DMD is caused by mutations to dystrophin that affect about 300,000 males worldwide & PTCs account for approximately 26% of cases.
2/Duchenne is a severe progressive disease which rapidly worsening children’s muscle function often using a wheelchair by early adolescence & eventually needing artificial ventilation to breathe. DMD is caused by mutations to dystrophin that affect about 300,000 males worldwide & PTCs account for approximately 26% of cases.

 2/IMO the most significant corporate event was $CRBU decision - due to internal portfolio prioritisation - to terminate the development of its CB-020 program - a preclinical allogeneic anti-POR1 CAR-NK cell therapy - a decision which narrowed dramatically the company’s pipeline.
2/IMO the most significant corporate event was $CRBU decision - due to internal portfolio prioritisation - to terminate the development of its CB-020 program - a preclinical allogeneic anti-POR1 CAR-NK cell therapy - a decision which narrowed dramatically the company’s pipeline. 

 2/@LineageCell has developed a unique technology that enables it to transplant specific cell types from a single pluripotent cell line thus creating “off the shelf” cell transplants platform for multiple conditions. $LCTX most advanced program is its OpRegen ocular platform. $XBI
2/@LineageCell has developed a unique technology that enables it to transplant specific cell types from a single pluripotent cell line thus creating “off the shelf” cell transplants platform for multiple conditions. $LCTX most advanced program is its OpRegen ocular platform. $XBI 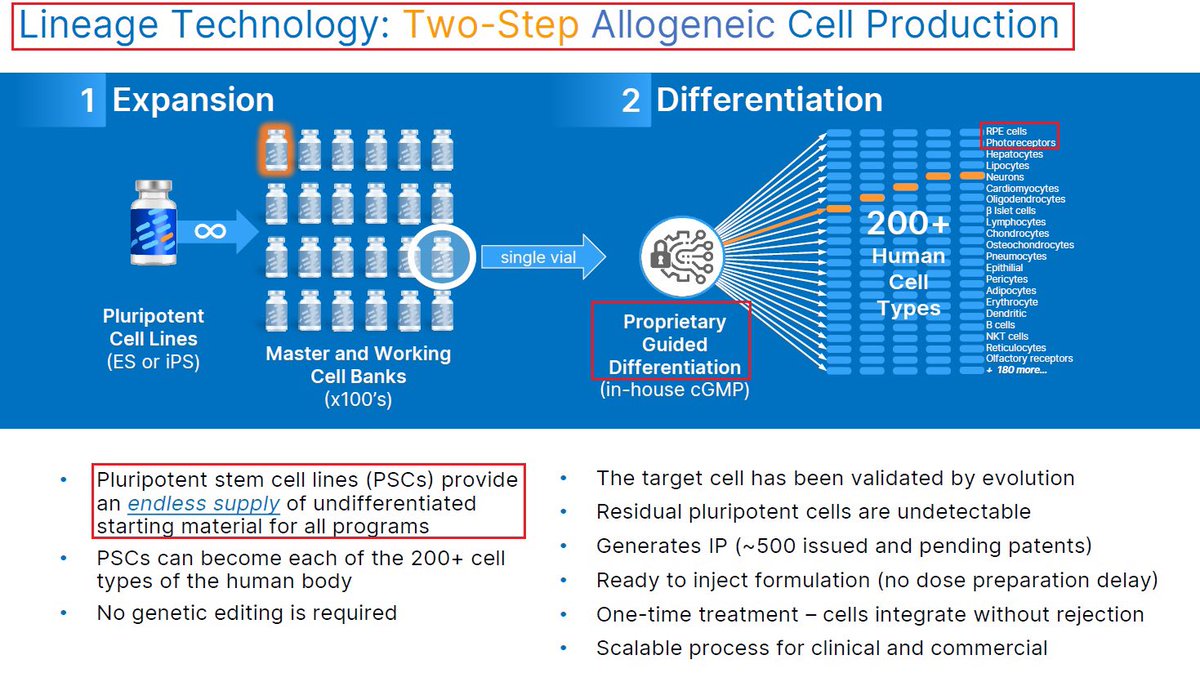

 2/WVE-006 is a first-in-class, GalNAc-conjugated RNA editing oligonucleotide aimed to correct the single base mutation in messenger RNA (mRNA) coded by the SERPINA1 Z allele, thereby enabling restoration and circulation of functional, wild-type alpha-1 antitrypsin (M-AAT) protein
2/WVE-006 is a first-in-class, GalNAc-conjugated RNA editing oligonucleotide aimed to correct the single base mutation in messenger RNA (mRNA) coded by the SERPINA1 Z allele, thereby enabling restoration and circulation of functional, wild-type alpha-1 antitrypsin (M-AAT) protein 

 2/VERVE-101 is being evaluated in the Heart-1 Phase 1b clinical trial with trial endpoints of safety and tolerability as well as changes in blood PCSK9 protein and low-density lipoprotein cholesterol (LDL-C) levels in patients living with heterozygous familial hypercholesterolemia (HeFH), established atherosclerotic cardiovascular disease (ASCVD), and uncontrolled hypercholesterolemia
2/VERVE-101 is being evaluated in the Heart-1 Phase 1b clinical trial with trial endpoints of safety and tolerability as well as changes in blood PCSK9 protein and low-density lipoprotein cholesterol (LDL-C) levels in patients living with heterozygous familial hypercholesterolemia (HeFH), established atherosclerotic cardiovascular disease (ASCVD), and uncontrolled hypercholesterolemia

 2/CB-012 is an anti-CLL-1 CAR-T cell therapy engineered with five genome edits, enabled by @CaribouBio’s patented next-generation CRISPR technology platform, which uses Cas12a chRDNA Gene Editing platform to significantly improve the specificity of genome edits. $CRBU
2/CB-012 is an anti-CLL-1 CAR-T cell therapy engineered with five genome edits, enabled by @CaribouBio’s patented next-generation CRISPR technology platform, which uses Cas12a chRDNA Gene Editing platform to significantly improve the specificity of genome edits. $CRBU 

 2/IMO the most significant corporate event was @VerveTx announcement that the @US_FDA has cleared its Investigational New Drug (IND) Application for $VERV-101 in patients with HeFH. Verve is currently working to activate U.S. trial sites & intends to dose the first patient with VERVE-101 in the US along with its ongoing UK & New Zealand clinical sites.
2/IMO the most significant corporate event was @VerveTx announcement that the @US_FDA has cleared its Investigational New Drug (IND) Application for $VERV-101 in patients with HeFH. Verve is currently working to activate U.S. trial sites & intends to dose the first patient with VERVE-101 in the US along with its ongoing UK & New Zealand clinical sites.

 2/IMO the most significant corporate event was @intelliatx collaboration signed with @ReCodeTx - which uses tissue-specific delivery to power mRNA & gene correction therapeutics to develop novel medicines for the treatment of Cystic fibrosis based on $NTLA Gene Editing platform
2/IMO the most significant corporate event was @intelliatx collaboration signed with @ReCodeTx - which uses tissue-specific delivery to power mRNA & gene correction therapeutics to develop novel medicines for the treatment of Cystic fibrosis based on $NTLA Gene Editing platform 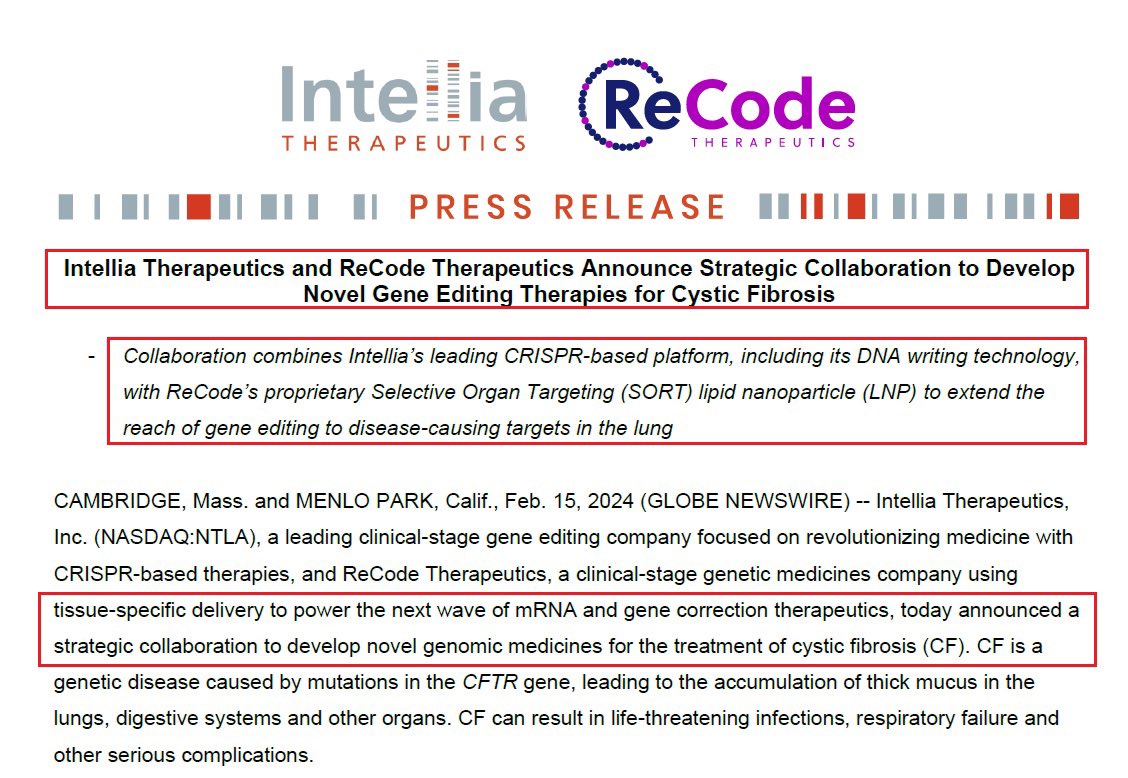

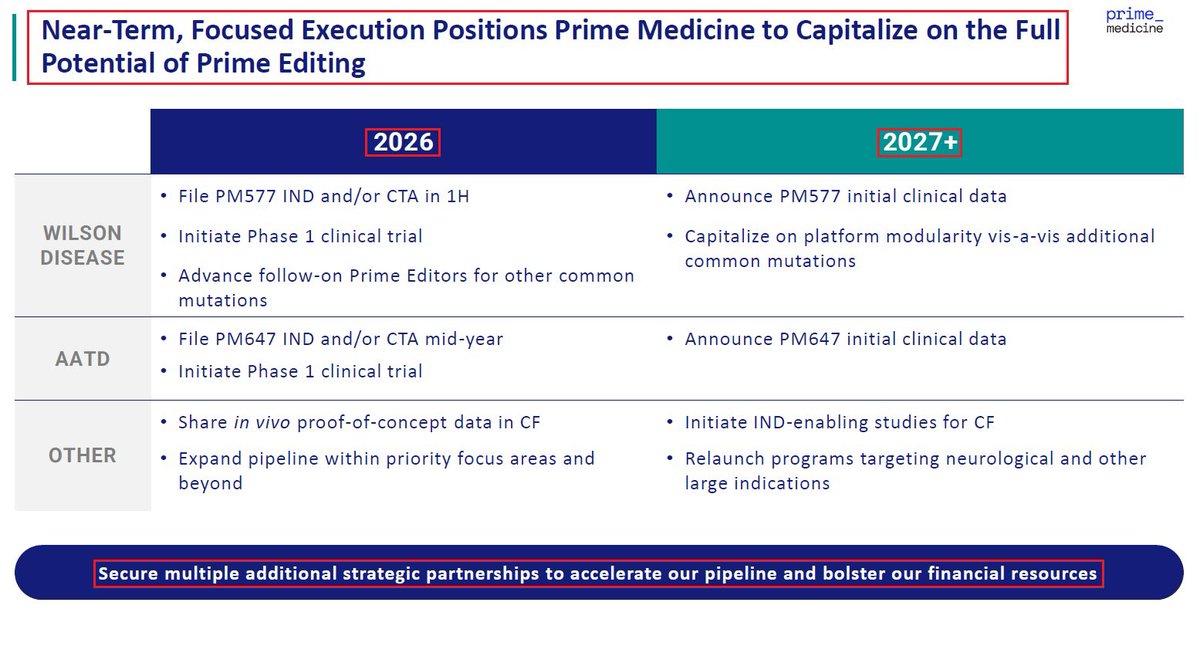
 2/PGI - programmable genomic integration - combines the site-specificity of #CRISPR/Cas9 with enzymes capable of inserting or writing sequences of DNA, including entire genes, without the need for double-strand DNA breaks. @Tome_bio’s most advanced PGI technology, called integrase-mediated PGI (I-PGI), utilizes proprietary integrases & is based on groundbreaking PASTE technology first discovered by Tome’s Co-
2/PGI - programmable genomic integration - combines the site-specificity of #CRISPR/Cas9 with enzymes capable of inserting or writing sequences of DNA, including entire genes, without the need for double-strand DNA breaks. @Tome_bio’s most advanced PGI technology, called integrase-mediated PGI (I-PGI), utilizes proprietary integrases & is based on groundbreaking PASTE technology first discovered by Tome’s Co-

 2/@PrimeMedicine’s proprietary platform - #PrimeEditing is the only #GeneEditing technology which can edit, correct, insert or even delete large DNA sequences in any target tissue - thus making it the most advanced & promising editing technology with $PRME owing its full rights
2/@PrimeMedicine’s proprietary platform - #PrimeEditing is the only #GeneEditing technology which can edit, correct, insert or even delete large DNA sequences in any target tissue - thus making it the most advanced & promising editing technology with $PRME owing its full rights 

 2/#RetinitisPigmentosa is a group of rare genetic eye diseases that damage light-sensitive cells in the retina thus leading to loss of sight. In ~10% of RP cases, the gene is passed from the mother to her children resulting in a form of #RP known as #XLRP.
2/#RetinitisPigmentosa is a group of rare genetic eye diseases that damage light-sensitive cells in the retina thus leading to loss of sight. In ~10% of RP cases, the gene is passed from the mother to her children resulting in a form of #RP known as #XLRP.

![Vertex Pharma announced today that the U.S. Food and Drug Administration (FDA) has approved CASGEVYTM (exagamglogene autotemcel [exa-cel]), a CRISPR/Cas9 gene-edited cell therapy, for the treatment of transfusion-dependent beta thalassemia (TDT) in patients 12 years and older](https://pbs.twimg.com/media/GD_iND7XgAAtDWg.jpg) 2/CASGEVYTM is a non-viral,ex vivo CRISPR/Cas9 gene-edited cell therapy for eligible patients with SCD or TDT, in which a patient’s own hematopoietic stem and progenitor cells are edited at the erythroid specific enhancer region of the BCL11A gene through a precise double-strand break.
2/CASGEVYTM is a non-viral,ex vivo CRISPR/Cas9 gene-edited cell therapy for eligible patients with SCD or TDT, in which a patient’s own hematopoietic stem and progenitor cells are edited at the erythroid specific enhancer region of the BCL11A gene through a precise double-strand break.