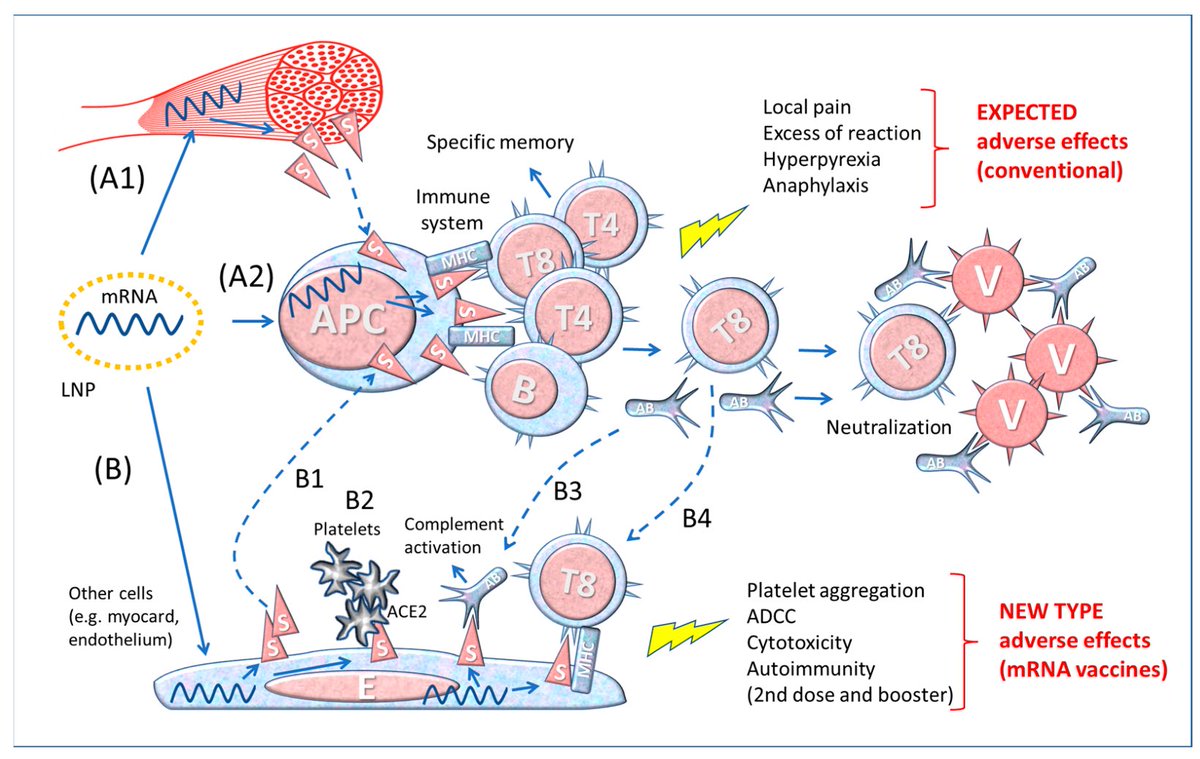
Why SARS-CoV-2 elicits mild symptoms at first but then, for a subset of patients, turn potentially fatal a week or so after infection? A recent study showed that distinct stages of illness correspond with the coronavirus acting differently in 2 different populations of cells 1/ 

The study’s findings may provide a roadmap for addressing cytokine storms and other excessive immune reactions that drive serious COVID-19. 2/ 

The team found that when SARS-2 infects its first-phase targets, cells in the lining of lung, two viral proteins circulate within those cells—one that works to activate the immune system & a 2nd that, paradoxically, blocks that signal, resulting in little or no inflammation 3/ 

A 2nd pathway the virus sometimes takes to enter immune cells. This alternative pathway both stunts virus's ability to reproduce & prevents production of the 2nd immune signal-braking protein. The 1st protein is then able to spur rampant inflammation linked to severe symptoms 4/
There are two stages that work through different signaling pathways. With the normal pathway, everything goes normally, and the virus replicates. When the immune cells pick up the virus, replication is defective, but it produces a lot of cytokines. 5/
In lab experiments, the researchers identified a drug that quelled inflammation in human immune cells infected with SARS-CoV-2 and reduced symptoms in mice, suggesting that it may be possible to prevent deadly cytokine storms. 6/ 

The researchers began with two related core questions: Why is so little inflammation seen with SARS-CoV-2 in lung cells? And, for the people who don't recover in the first seven to 10 days, why do the symptoms get so much worse? 7/
They started by screening all SARS-CoV-2 proteins to see which ones regulate the production of cytokines. Their search yielded two results: a protein called NSP14, which causes inflammation, and another called ORF6, which quells it. 8/ 

The team followed up on this finding with an exhaustive series of experiments encompassing protein-on-protein reactions, human and animal cell lines, lab models, and tissue and fluid samples from COVID-19 patients. 9/
This is the picture that emerged: When the coronavirus enters a cell of the lung lining through its known entry point, the ACE2 protein on the cell's surface, both NSP14 and ORF6, are produced within the cell. 10/
ORF6 acts as a guardian at the membrane surrounding the cell nucleus, effectively silencing the inflammatory signal. 11/
In this population of lung cells, ORF6 overrides NSP14 so that no cytokines are produced. Even if NSP14 takes action, ORF6 doesn't let it get into the cell nucleus. The door is closed, so it's completely blocked for cytokine production. 12/
So, a strong viral replication but scarce inflammatory response during the early (ACE2-dependent) infection stage, followed by low viral replication & potent inflammatory response in the late (ACE2-independent) infection stage, may contribute to COVID-19 progression. 13/13
• • •
Missing some Tweet in this thread? You can try to
force a refresh