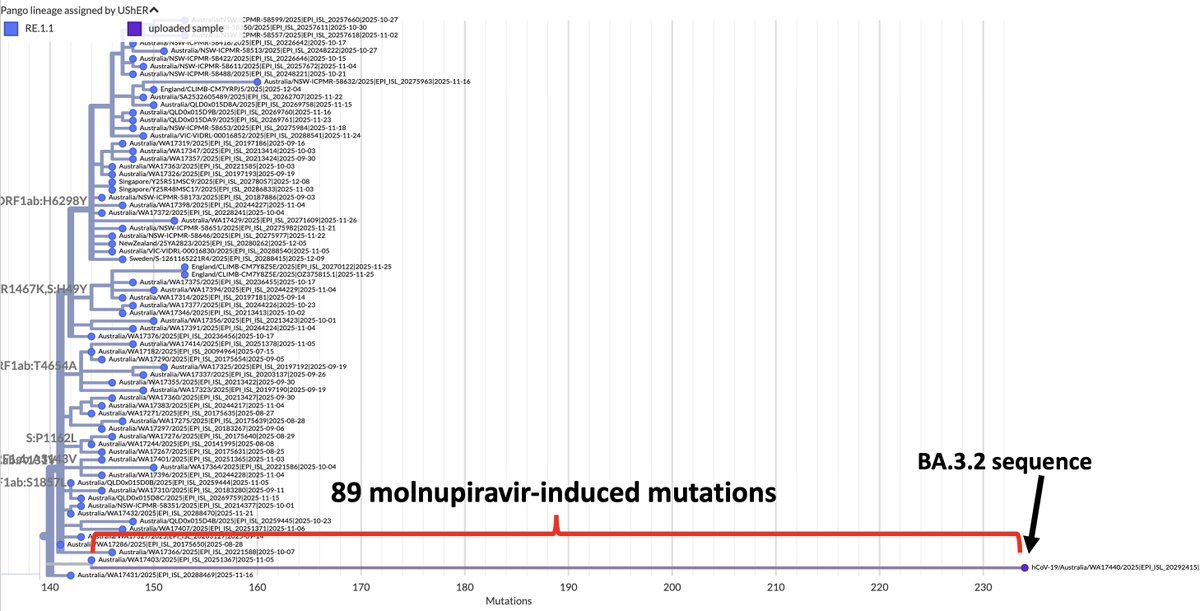
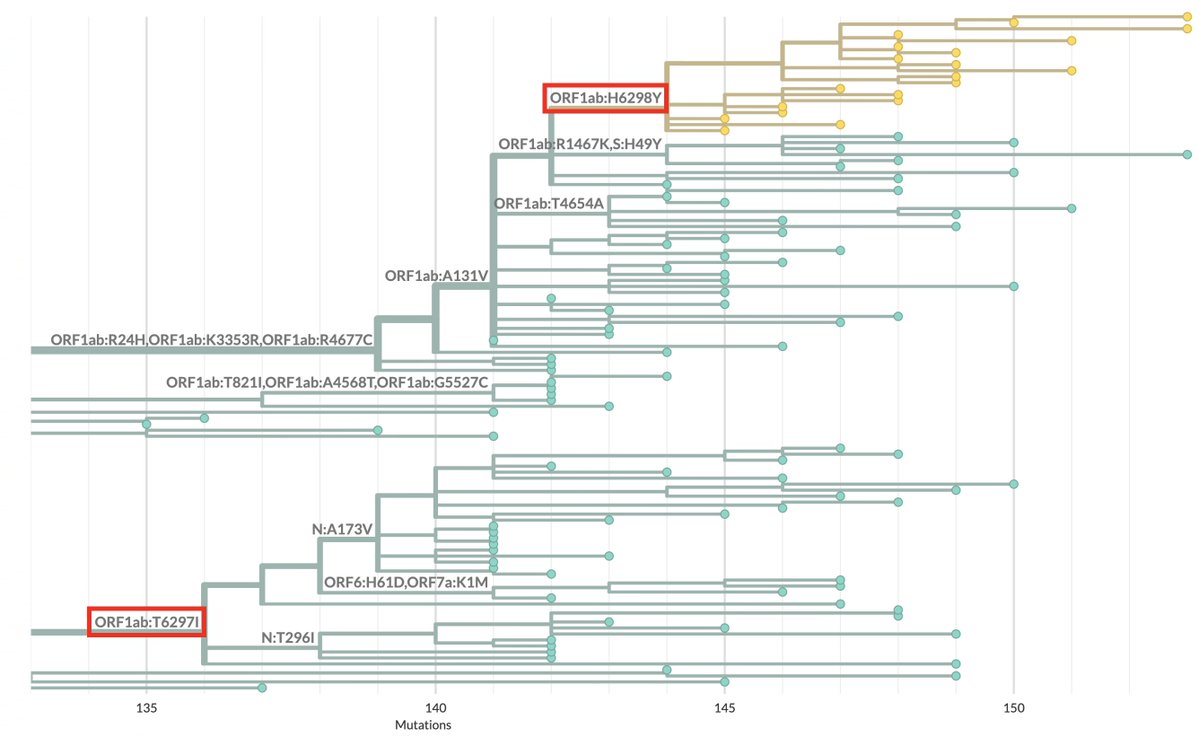
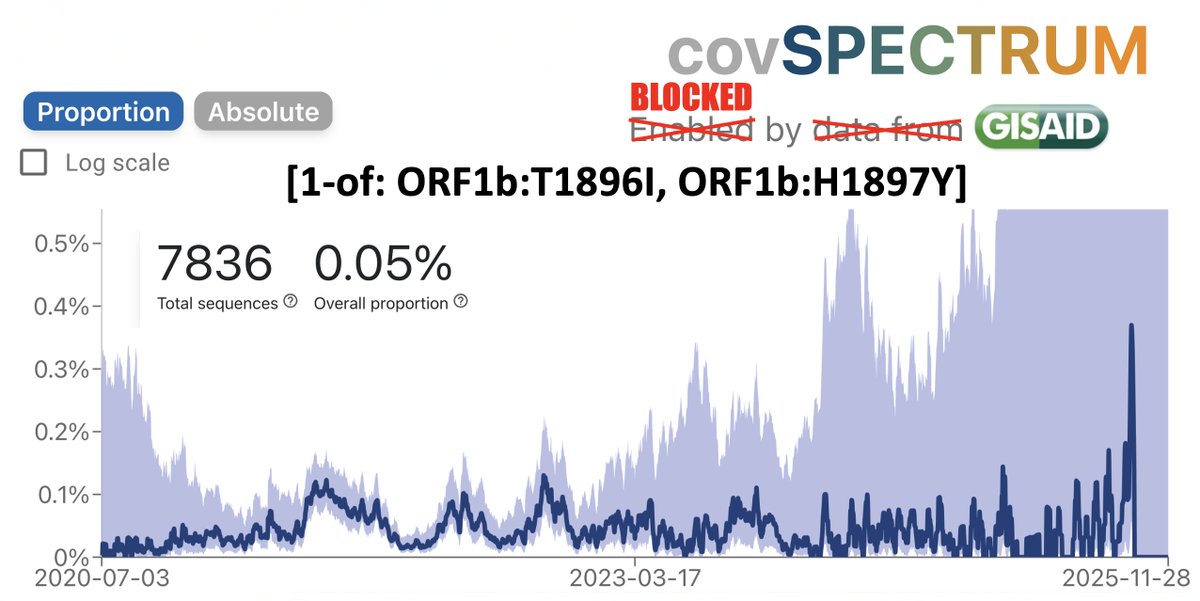
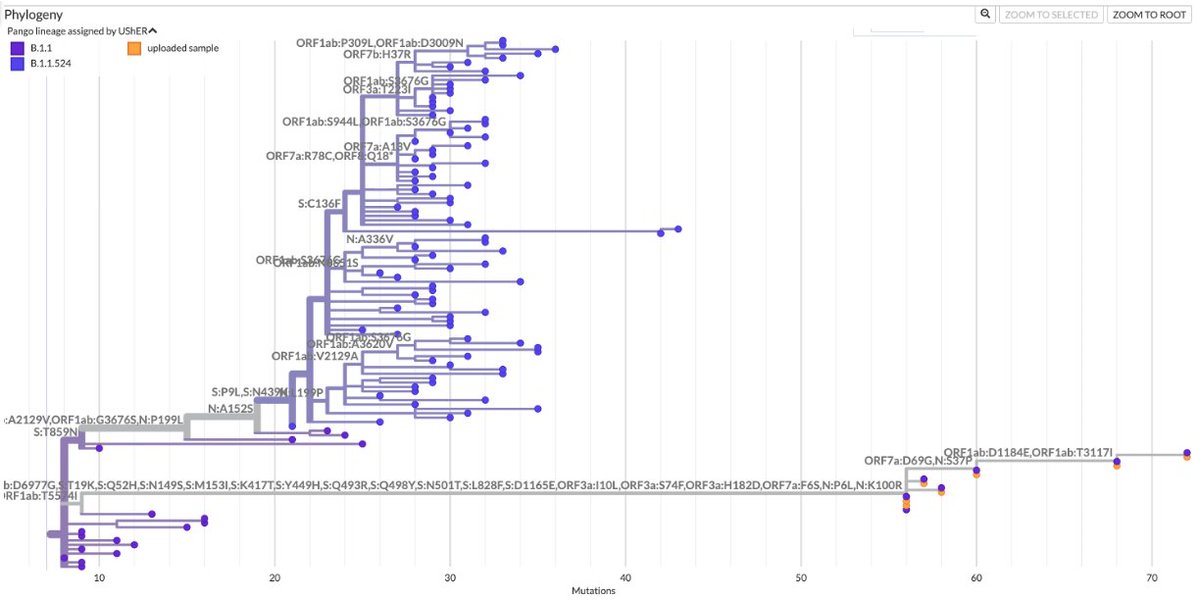
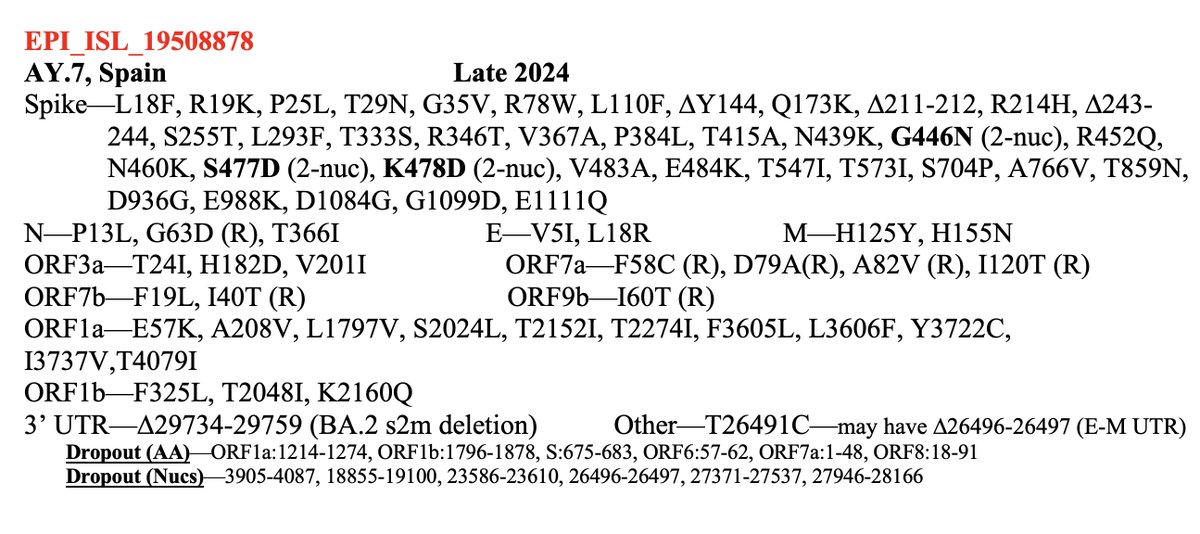
Always nice to run across a possible function of a rare mutation that's shown up in multiple chronic-infection SARS-CoV-2 seqs. Thanks to an excellent paper by @TheMenacheryLab & @J_Paul_Taylor, I think I now know why N:L13P (a reversion) shows up. 1/6
https://x.com/TheMenacheryLab/status/1770959155093705059
They proved that the N:1-25 region, esp. the ITFG AA motif from N:15-18, is the essential element in N's ability to suppress the formation of stress granules (SGs) in cells, which capture & disable long viral RNAs & help organizing innate antiviral immune responses. 2/6 



All variants retain the ability to suppress SGs, but Omicron's N:P13L weakens N's binding to G3BP1/2—the master cellular regulators of SGs—by about 2.3-fold. That's pretty slight, & almost certainly not enough selection pressure to result in reversions in circulation... 3/6 

...but >1-year-long infections, w/no transmission bottleneck, can select for slightly advantageous mutations that never have a chance to emerge via stepwise evolution.
Of course, there must have been some advantage for N:P13L to evolve in Omicron (& other lineages). 4/6
Of course, there must have been some advantage for N:P13L to evolve in Omicron (& other lineages). 4/6
One possible reason was pointed out to me by @PeacockFlu: for one HLA haplotype (D*), N:9-17 is a potent T-cell epitope. 5/6 onlinelibrary.wiley.com/doi/10.1002/ct…

It's a pretty small thing—not like when @SolidEvidence & @wanderer_jasnah deciphered the ORF1a:K1795Q story—but when you've run up against countless genetic enigmas & inscrutable oddities, it's nice to have a (tentative) answer of any sort. I'll take a small, good thing. 6/6
Went to look up the HLA haplotype for the N:9-17 T-cell epitope & then forgot to fill it in in tweet #5. It should say B*:27:05.
More evidence Omicron's N:P13L is a factor from a paper I just read today.
"In contrast to [WT] & Delta, Omicron strongly induced SG formation, especially ...late in infection."
ISR = integrated stress response. Among other things, provokes SG formation.
ISRIB = ISR inhibitor
"In contrast to [WT] & Delta, Omicron strongly induced SG formation, especially ...late in infection."
ISR = integrated stress response. Among other things, provokes SG formation.
ISRIB = ISR inhibitor

Link to that study: pubmed.ncbi.nlm.nih.gov/37979658/
@threadreaderapp unroll
• • •
Missing some Tweet in this thread? You can try to
force a refresh