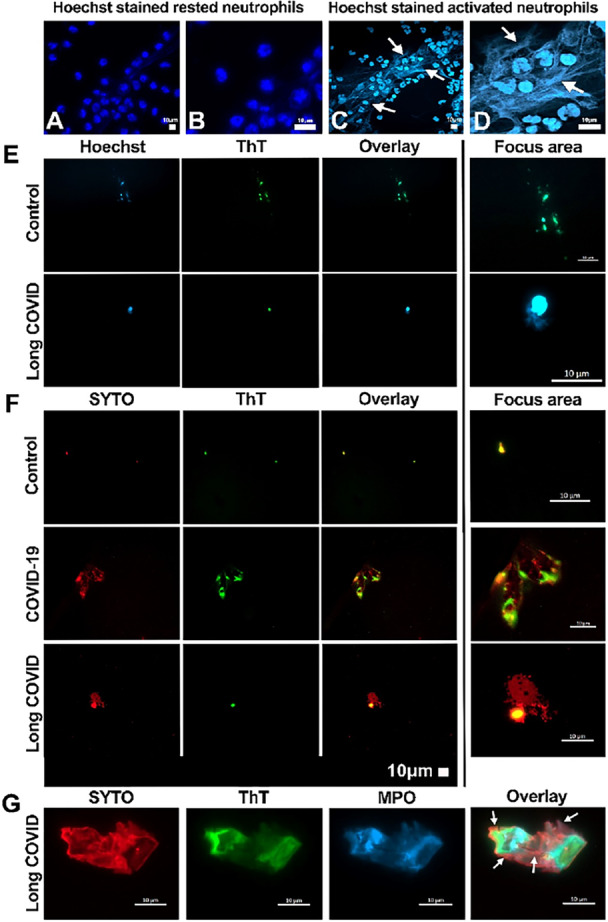
𝙄𝙣𝙛𝙡𝙖𝙢𝙢𝙖𝙩𝙞𝙤𝙣 𝙞𝙣 𝙋𝘼𝙎𝘾-𝘾𝙑𝙎 𝙞𝙣𝙙𝙞𝙫𝙞𝙙𝙪𝙖𝙡𝙨 >18 𝙢𝙤𝙣𝙩𝙝𝙨 𝙥𝙤𝙨𝙩-𝙞𝙣𝙛𝙚𝙘𝙩𝙞𝙤𝙣 𝙢𝙖𝙮 𝙙𝙧𝙞𝙫𝙚 𝙘𝙖𝙧𝙙𝙞𝙤𝙫𝙖𝙨𝙘𝙪𝙡𝙖𝙧 𝙨𝙮𝙢𝙥𝙩𝙤𝙢𝙨 𝙩𝙝𝙧𝙤𝙪𝙜𝙝 𝙚𝙛𝙛𝙚𝙘𝙩𝙨 𝙤𝙣 𝙘𝙖𝙧𝙙𝙞𝙤𝙢𝙮𝙤𝙘𝙮𝙩𝙚 𝙛𝙪𝙣𝙘𝙩𝙞𝙤𝙣
biorxiv.org/content/10.110…
biorxiv.org/content/10.110…
2) The study investigated individuals with post-acute sequelae of COVID-19 cardiovascular syndrome (PASC-CVS), defined as persistent chest pain and/or heart palpitations more than 12 weeks after SARS-CoV-2 infection. 
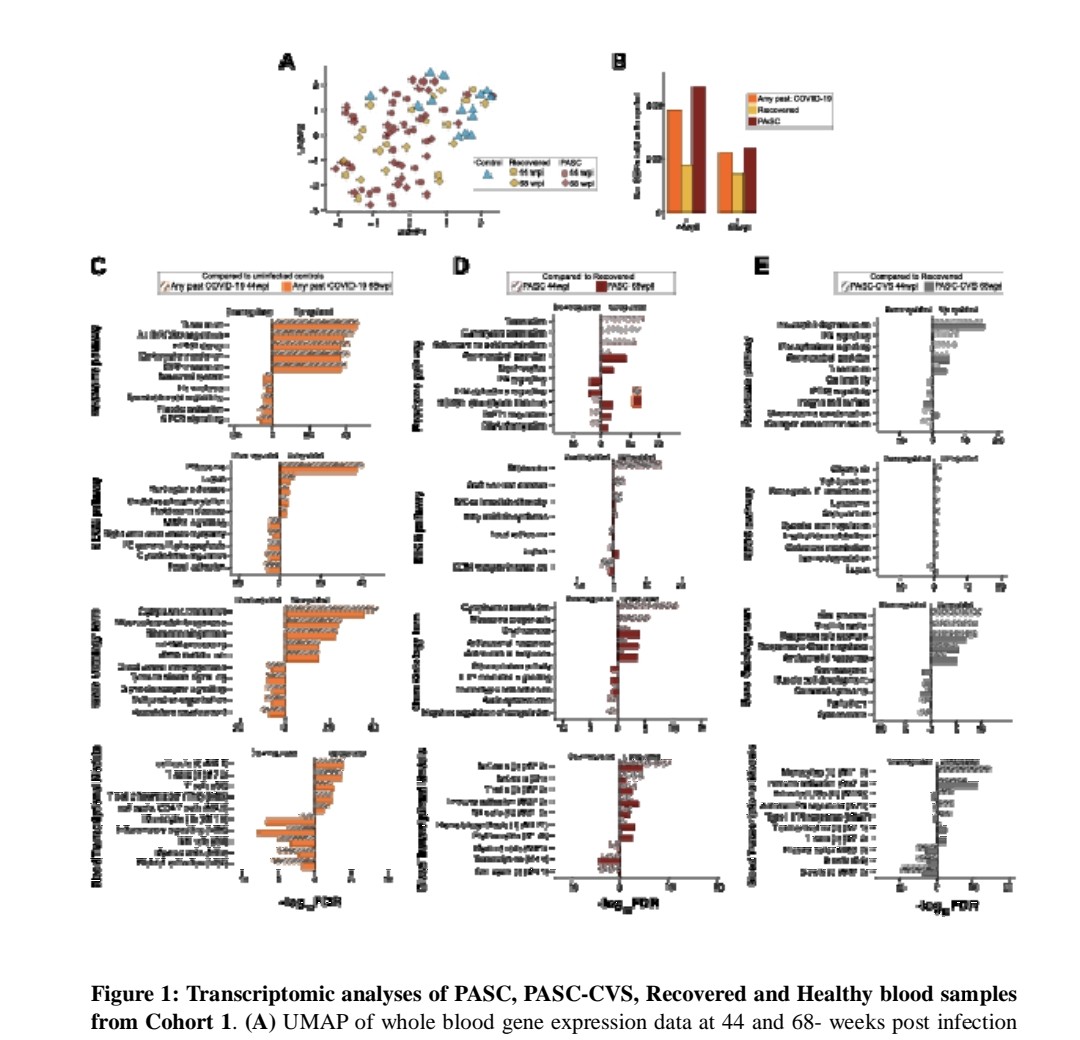
3) Two independent cohorts of PASC-CVS individuals were compared to individuals who recovered from COVID-19 without long-term symptoms.
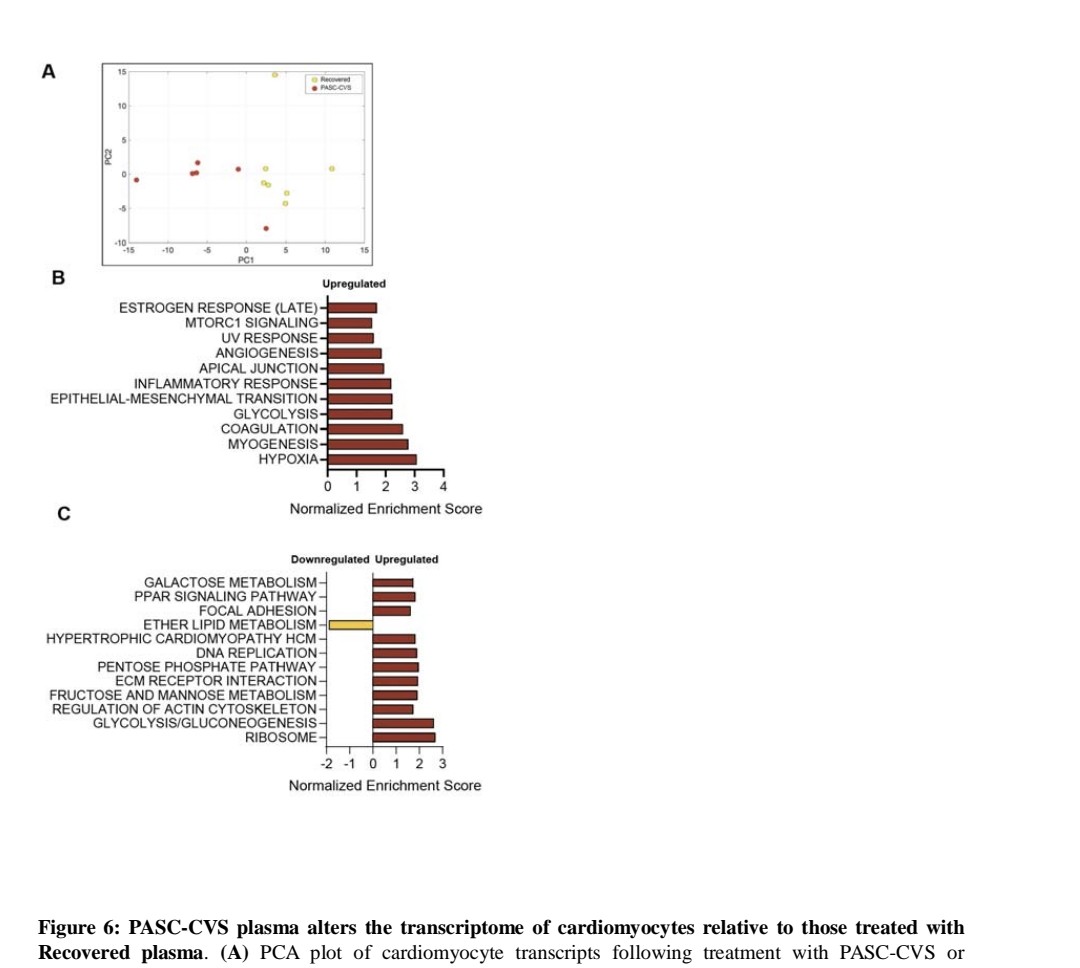
Whole blood RNA sequencing showed PASC-CVS donors had an upregulated inflammatory transcriptional signature over 500 days post-infection ...
Whole blood RNA sequencing showed PASC-CVS donors had an upregulated inflammatory transcriptional signature over 500 days post-infection ...
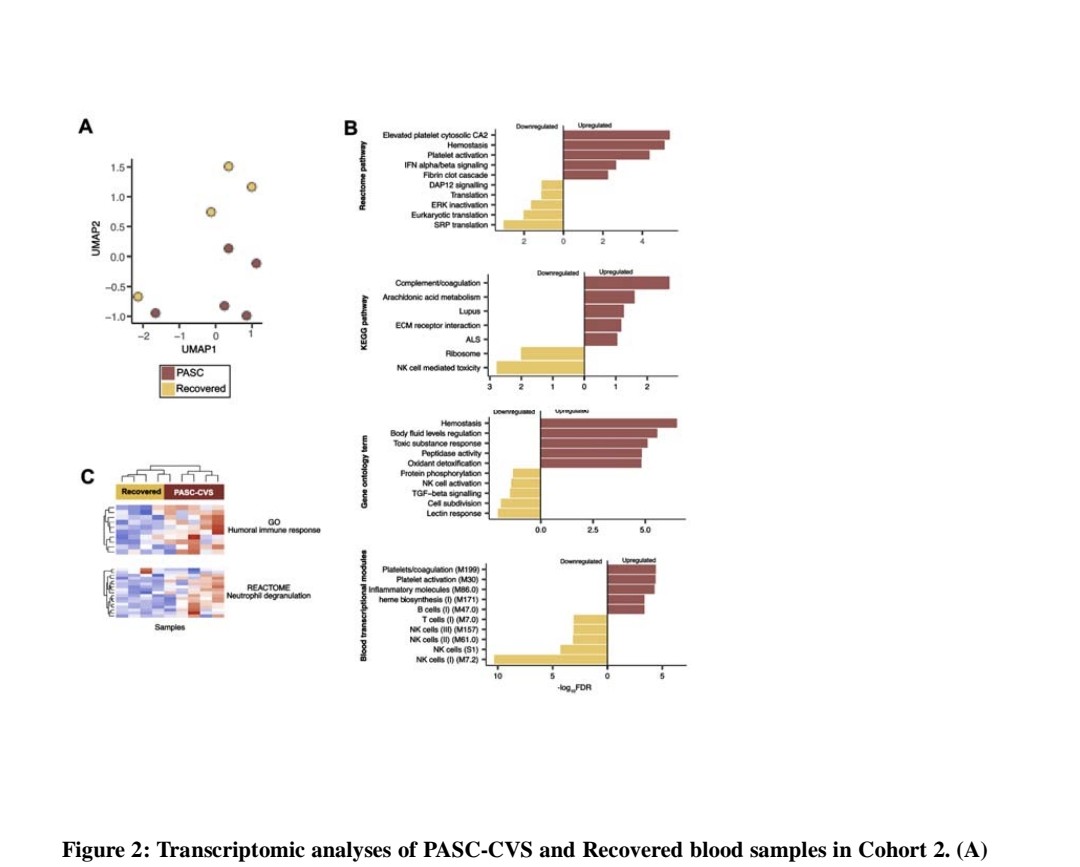
4) ...with enrichment of neutrophil degranulation and interferon pathways.
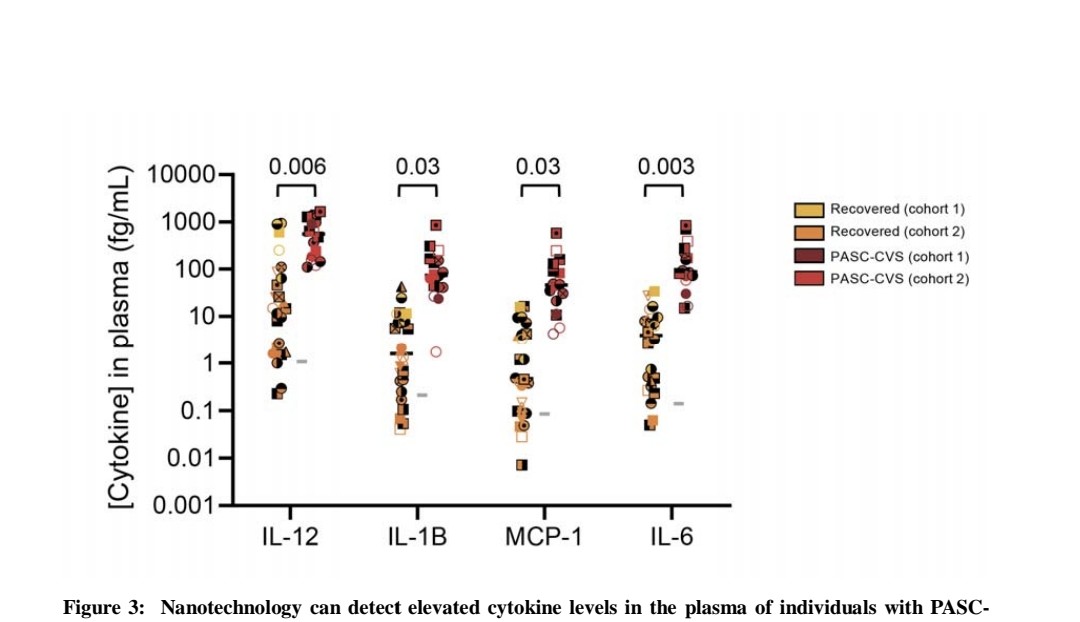
Nanotechnology (immunostorm chip) detected significantly elevated trace levels of IL-12, IL-1β, MCP-1 and IL-6 in PASC-CVS plasma compared to Recovered donors.
Nanotechnology (immunostorm chip) detected significantly elevated trace levels of IL-12, IL-1β, MCP-1 and IL-6 in PASC-CVS plasma compared to Recovered donors.
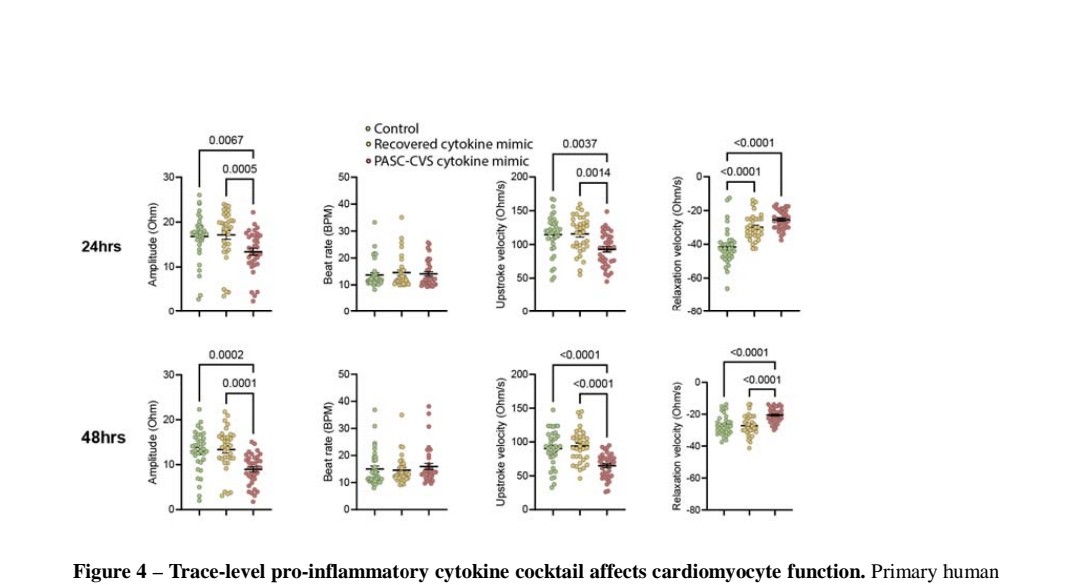
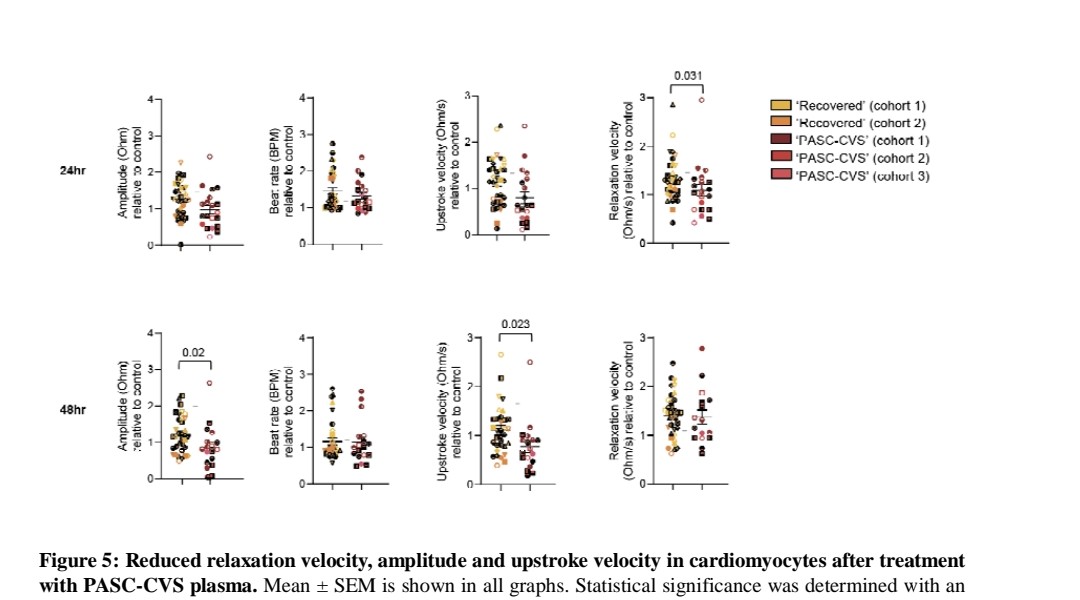
5) A cytokine cocktail mimicking PASC-CVS levels impaired cardiomyocyte function in vitro, reducing amplitude and upstroke velocity. PASC-CVS plasma also reduced cardiomyocyte relaxation velocity.
Proteomics of PASC-CVS plasma found enrichment of complement cascade and ...
Proteomics of PASC-CVS plasma found enrichment of complement cascade and ...
6) ... and serum amyloid A-4.
Treatment with dexamethasone prevented the effect of PASC-CVS plasma on cardiomyocyte upstroke velocity.
Treatment with dexamethasone prevented the effect of PASC-CVS plasma on cardiomyocyte upstroke velocity.
7) The results suggest chronic low-level inflammation in PASC-CVS individuals over 18 months post-infection may drive cardiovascular symptoms through effects on cardiomyocyte function. Nanotechnology showed potential for improved PASC-CVS diagnosis and management.
Thanks 🙏
Thanks 🙏
• • •
Missing some Tweet in this thread? You can try to
force a refresh