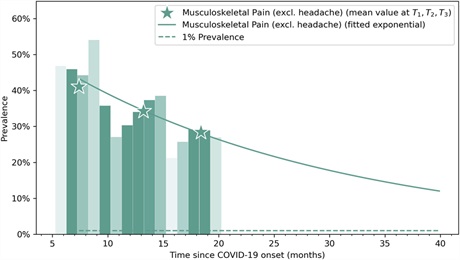
𝗖𝗢𝗩𝗜𝗗 𝗜𝗡𝗙𝗘𝗖𝗧𝗜𝗢𝗡 & 𝗟𝗢𝗡𝗚 𝗖𝗢𝗩𝗜𝗗
𝗪𝗵𝗶𝗰𝗵 𝗼𝗿𝗴𝗮𝗻𝘀, 𝘀𝘆𝘀𝘁𝗲𝗺𝘀 𝗮𝗿𝗲 𝗶𝗺𝗽𝗮𝗰𝘁𝗲𝗱 ? 𝗪𝗵𝗶𝗰𝗵 𝘀𝘆𝗺𝗽𝘁𝗼𝗺𝘀 ?
The pathophysiology of infection and PASC is complex and multifactorial, involving direct organ damage from virus infection ..
𝗪𝗵𝗶𝗰𝗵 𝗼𝗿𝗴𝗮𝗻𝘀, 𝘀𝘆𝘀𝘁𝗲𝗺𝘀 𝗮𝗿𝗲 𝗶𝗺𝗽𝗮𝗰𝘁𝗲𝗱 ? 𝗪𝗵𝗶𝗰𝗵 𝘀𝘆𝗺𝗽𝘁𝗼𝗺𝘀 ?
The pathophysiology of infection and PASC is complex and multifactorial, involving direct organ damage from virus infection ..
2) ...immune system dysregulation, autoimmune responses, microthrombosis, and persistent viral proteins/inflammation.
Multiple functions and organs are commonly impacted include lungs (pulmonary fibrosis, vascular dysfunction), heart (myocarditis, microvascular damage),
Multiple functions and organs are commonly impacted include lungs (pulmonary fibrosis, vascular dysfunction), heart (myocarditis, microvascular damage),
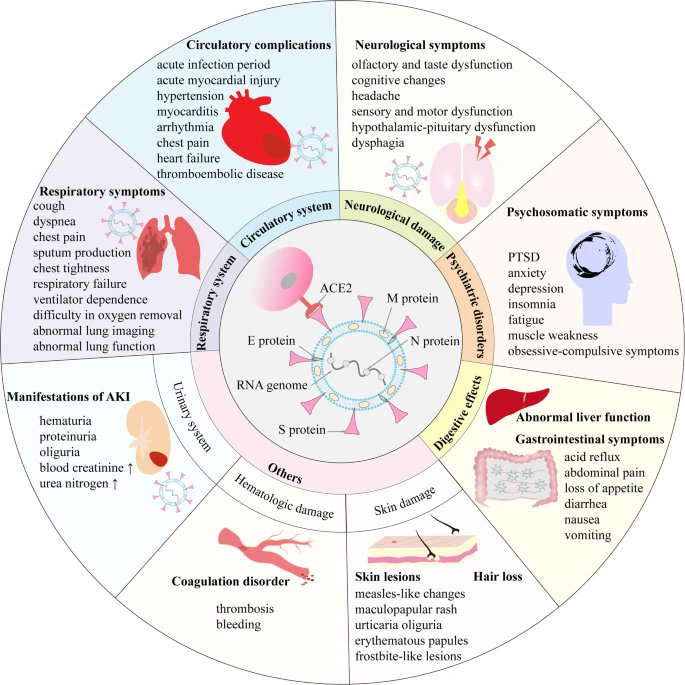
3) ...brain (neuroinflammation, autonomic dysfunction), gut (microbiome changes), kidneys, blood (hypercoagulability), and skin.
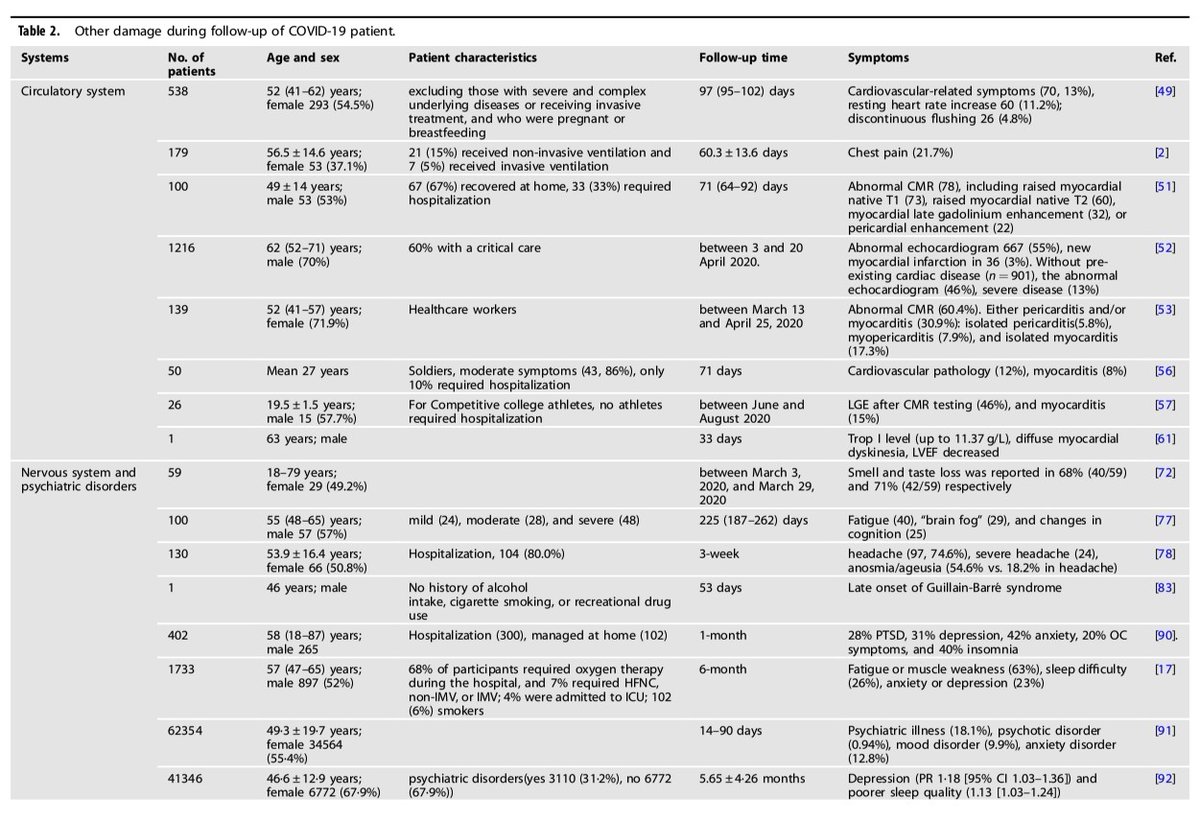
In these tables, there are the systems affected, the number of patients, their characteristics, symptoms and so many reference studies.
In these tables, there are the systems affected, the number of patients, their characteristics, symptoms and so many reference studies.
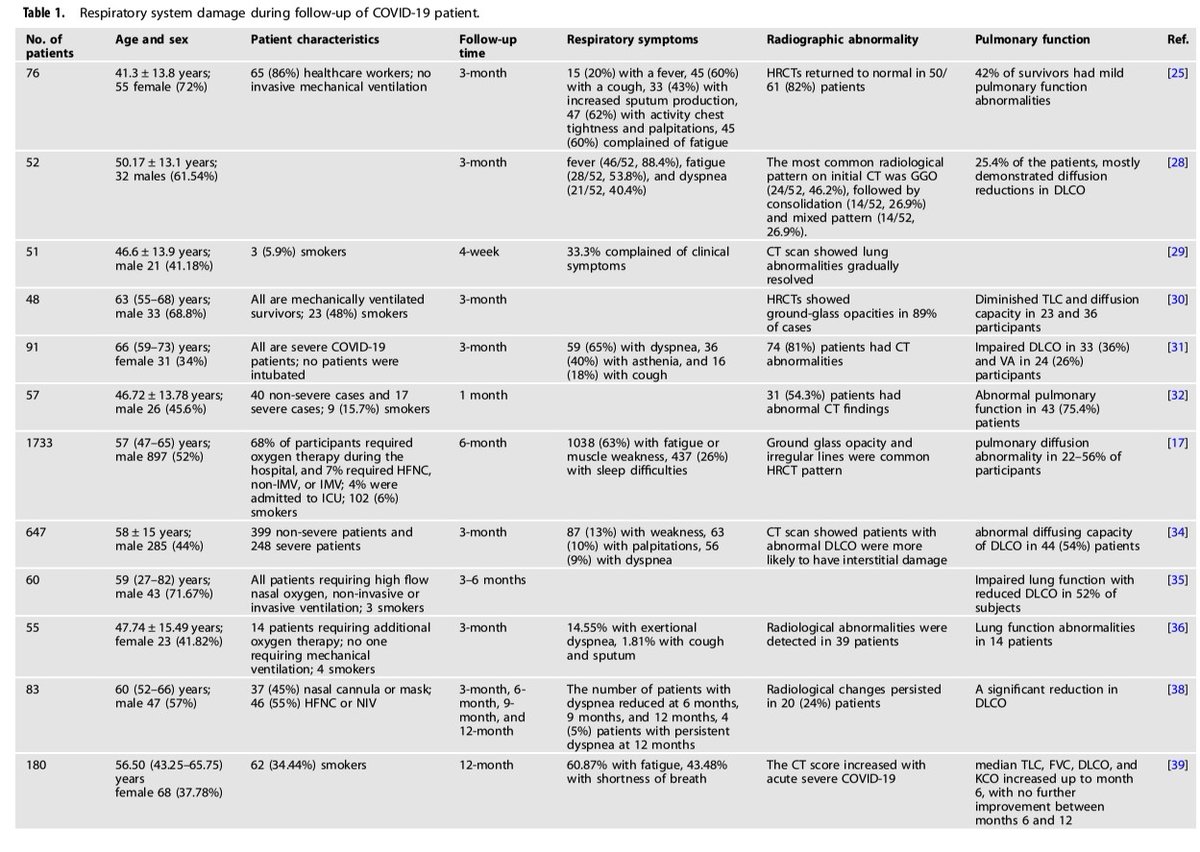
4) We have decided in this thread to summarize partially, these 2 tables with affected systems, nb of studies, nb of patients and symptoms, to visualise to what extent COVID-19 can impact our body. 
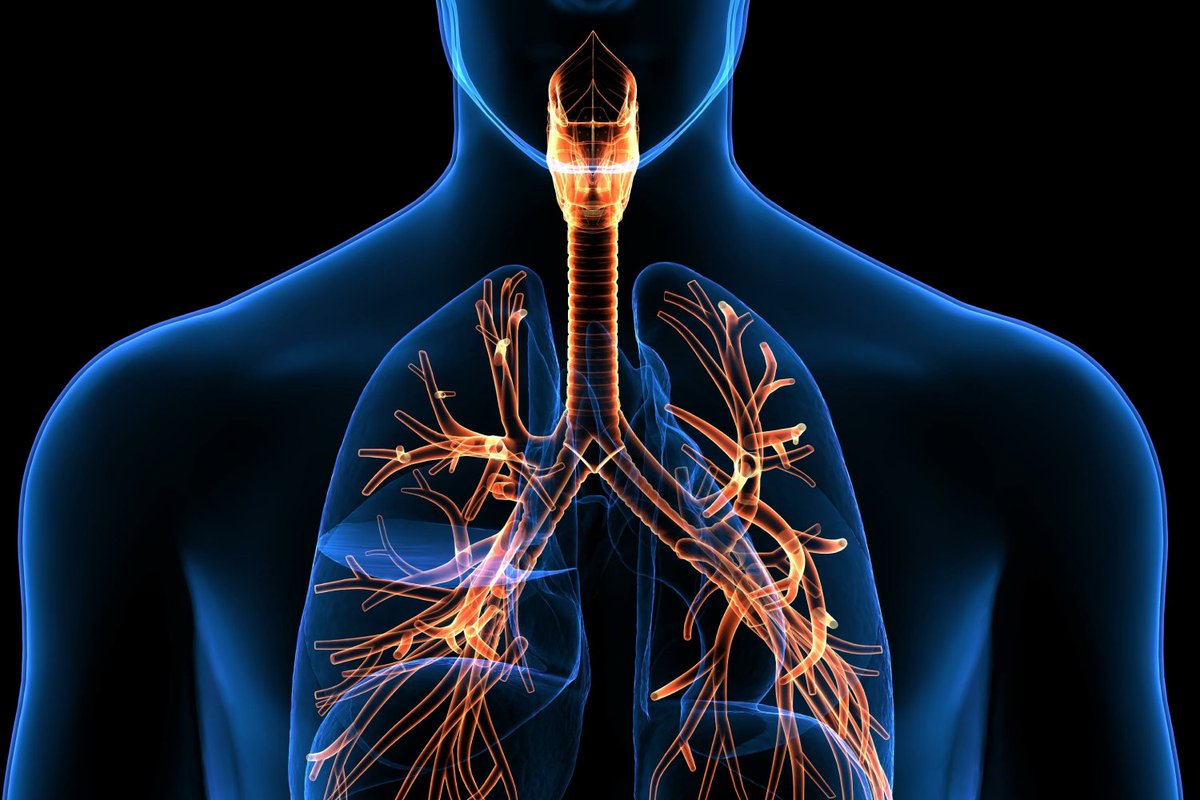
5) 𝙍𝙀𝙎𝙋𝙄𝙍𝘼𝙏𝙊𝙍𝙔 𝙎𝙔𝙎𝙏𝙀𝙈
12 studies, more than 3.000 patients, follow-up 3-12 months, symptoms :
▶️ 42% of survivors had mild pulmonary function abnormalities
▶️ 25.4% of the patients, mostly demonstrated diffusion reductions in DLCO
...
12 studies, more than 3.000 patients, follow-up 3-12 months, symptoms :
▶️ 42% of survivors had mild pulmonary function abnormalities
▶️ 25.4% of the patients, mostly demonstrated diffusion reductions in DLCO
...
6)
▶️ Diminished TLC and diffusion capacity in 23 and 36 participants
▶️ Impaired DLCO in 33 (36%) and VA in 24 (26%) participants
▶️ Abnormal pulmonary function in 43 (75.4%) patients
▶️ Pulmonary diffusion abnormality in 22-56% of participants
...
▶️ Diminished TLC and diffusion capacity in 23 and 36 participants
▶️ Impaired DLCO in 33 (36%) and VA in 24 (26%) participants
▶️ Abnormal pulmonary function in 43 (75.4%) patients
▶️ Pulmonary diffusion abnormality in 22-56% of participants
...
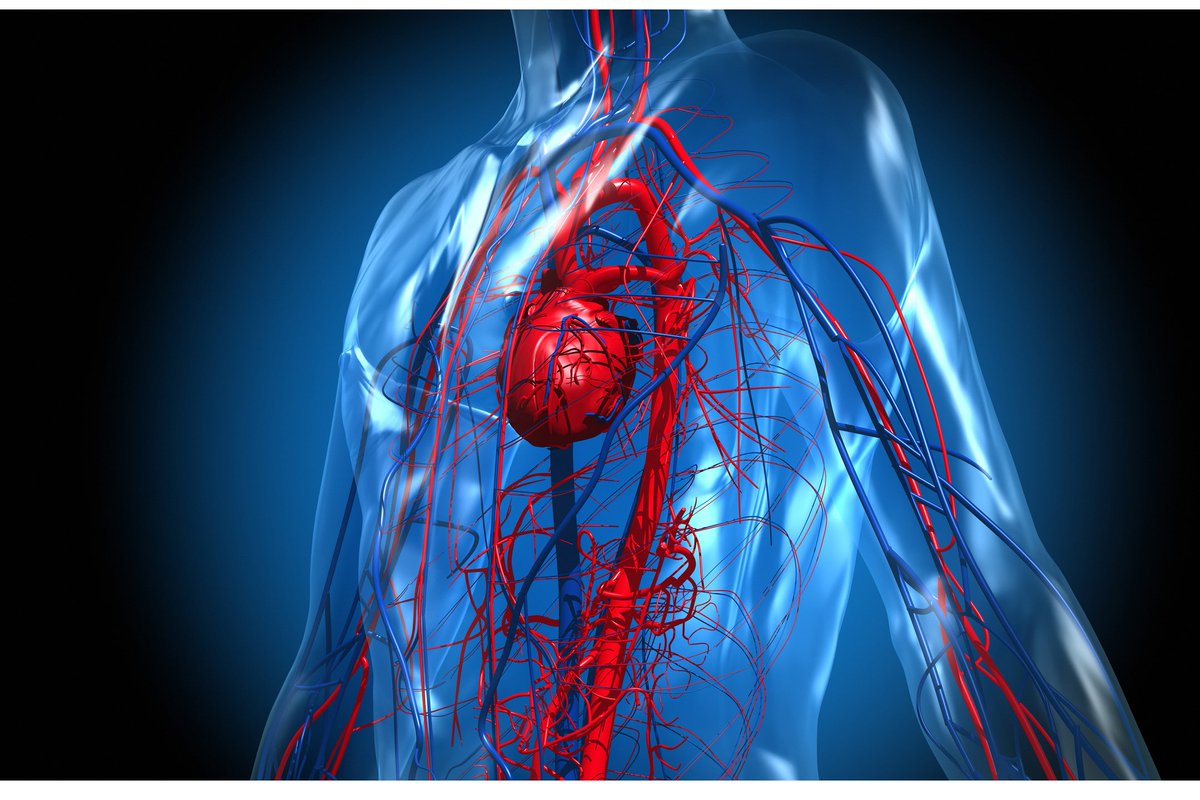
7) 𝘾𝙄𝙍𝘾𝙐𝙇𝘼𝙏𝙊𝙍𝙔 𝙎𝙔𝙎𝙏𝙀𝙈
7 studies, more than 2.000 patients, follow-up 17-97 days, symptoms :
▶️ Cardiovascular-related symptoms (70, 13%), resting heart rate increase 60 (11.2%)
▶️ Chest pain (21.7%)
▶️ Abnormal CMR (78), including raised myocardial native T1 (73)
7 studies, more than 2.000 patients, follow-up 17-97 days, symptoms :
▶️ Cardiovascular-related symptoms (70, 13%), resting heart rate increase 60 (11.2%)
▶️ Chest pain (21.7%)
▶️ Abnormal CMR (78), including raised myocardial native T1 (73)
8)
▶️ Abnormal echocardiogram 667 (55%), new myocardial infarction in 36 (3%). ▶️ Abnormal CMR (60.4%). Either pericarditis and/or myocarditis (30.9%): isolated pericarditis (5.8%), myopericarditis (7.9%),
▶️ Cardiovascular pathology (12%), myocarditis (8%)
...
▶️ Abnormal echocardiogram 667 (55%), new myocardial infarction in 36 (3%). ▶️ Abnormal CMR (60.4%). Either pericarditis and/or myocarditis (30.9%): isolated pericarditis (5.8%), myopericarditis (7.9%),
▶️ Cardiovascular pathology (12%), myocarditis (8%)
...
9) 𝙉𝙀𝙍𝙑𝙊𝙐𝙎 𝙎𝙔𝙎𝙏𝙀𝙈 𝙖𝙣𝙙 𝙋𝙎𝙔𝘾𝙃𝙄𝘼𝙏𝙍𝙄𝘾 𝘿𝙄𝙎𝙊𝙍𝘿𝙀𝙍𝙎
8 studies, more than 100.000 patients, follow-up 15 days-6 months, symptoms :
▶️ Smell and taste loss was reported in 68% (40/59)
▶️ Fatigue (40), "brain fog" (29), and changes in cognition (25)
8 studies, more than 100.000 patients, follow-up 15 days-6 months, symptoms :
▶️ Smell and taste loss was reported in 68% (40/59)
▶️ Fatigue (40), "brain fog" (29), and changes in cognition (25)
10) ▶️ Headache (97, 74.6%), severe headache (24), anosmia/ageusia (54.6% vs. 18.2% in headache)
▶️ Late onset of Guillain-Barré syndrome
▶️ 28% PTSD, 31% depression, 42% anxiety, 20% OC symptoms, and 40% insomnia
▶️ Psychiatric illness (18.1%), mood disorder (9.9%)
...
▶️ Late onset of Guillain-Barré syndrome
▶️ 28% PTSD, 31% depression, 42% anxiety, 20% OC symptoms, and 40% insomnia
▶️ Psychiatric illness (18.1%), mood disorder (9.9%)
...
11) There is of course much more information in this fascinating study that we highly recommend.
H/t @inkblue01
Thanks for reading 🙏 nature.com/articles/s4141…

H/t @inkblue01
Thanks for reading 🙏 nature.com/articles/s4141…

• • •
Missing some Tweet in this thread? You can try to
force a refresh