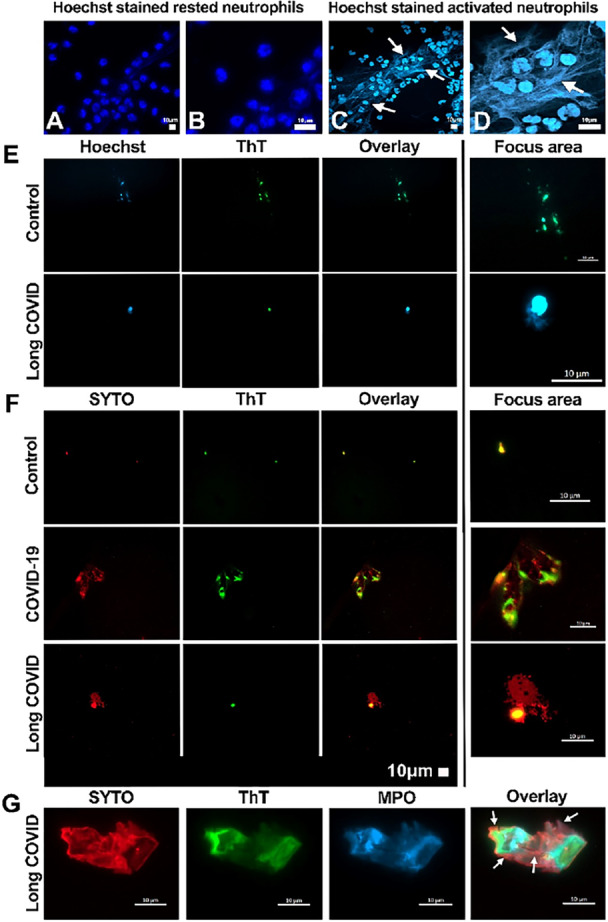
▶️ 𝐊𝐄𝐘 𝐒𝐓𝐔𝐃𝐘 : 👍
▶️ 𝐊𝐄𝐘 𝐌𝐔𝐓𝐀𝐓𝐈𝐎𝐍 : 𝐄𝐓9𝐈
▶️ 𝐊𝐄𝐘 𝐒𝐀𝐑𝐒-𝐂𝐎𝐕-2 𝐏𝐑𝐎𝐓𝐄𝐈𝐍 : 𝐄𝐍𝐕𝐄𝐋𝐎𝐏𝐄
Being one of the most important studies we have read, we will take the time to explain all the concepts, one by one.
▶️ 𝐊𝐄𝐘 𝐌𝐔𝐓𝐀𝐓𝐈𝐎𝐍 : 𝐄𝐓9𝐈
▶️ 𝐊𝐄𝐘 𝐒𝐀𝐑𝐒-𝐂𝐎𝐕-2 𝐏𝐑𝐎𝐓𝐄𝐈𝐍 : 𝐄𝐍𝐕𝐄𝐋𝐎𝐏𝐄
Being one of the most important studies we have read, we will take the time to explain all the concepts, one by one.

2) 𝙒𝙝𝙖𝙩 𝙖𝙧𝙚 𝘼𝙪𝙩𝙤𝙥𝙝𝙖𝙜𝙤𝙨𝙤𝙢𝙚𝙨 ?
Autophagosomes are double-membrane vesicles formed during autophagy, that sequester cytoplasmic cargo like proteins, organelles and pathogens targeted for degradation.
Upon SARS-CoV-2 invasion, host cells induce autophagy ...
Autophagosomes are double-membrane vesicles formed during autophagy, that sequester cytoplasmic cargo like proteins, organelles and pathogens targeted for degradation.
Upon SARS-CoV-2 invasion, host cells induce autophagy ...

3) ...as an intrinsic antiviral defense mechanism. Autophagosomes capture invading viral particles and components.
Cargo-loaded autophagosomes then fuse with lysosomes. This degrades intact virions and disrupts infection.
Cargo-loaded autophagosomes then fuse with lysosomes. This degrades intact virions and disrupts infection.

4) 𝙒𝙝𝙖𝙩 𝙖𝙧𝙚 𝙇𝙮𝙨𝙤𝙨𝙤𝙢𝙚𝙨 ?
Lysosomes are membrane-bound organelles that contain digestive enzymes and maintain an acidic pH optimal for degradation. As part of the autophagy response, SARS-CoV-2 virions and viral components that are sequestered inside autophagosomes
Lysosomes are membrane-bound organelles that contain digestive enzymes and maintain an acidic pH optimal for degradation. As part of the autophagy response, SARS-CoV-2 virions and viral components that are sequestered inside autophagosomes

5) ...are delivered to lysosomes upon fusion.
The acidic pH and hydrolases inside lysosomes promote destruction of viral structural proteins, genetic material, lipids etc. This degrades and destroys intact virions.
The acidic pH and hydrolases inside lysosomes promote destruction of viral structural proteins, genetic material, lipids etc. This degrades and destroys intact virions.

6) 𝙒𝙝𝙖𝙩 𝙞𝙨 𝙇𝙮𝙨𝙤𝙨𝙤𝙢𝙖𝙡 𝙙𝙚𝙜𝙧𝙖𝙙𝙖𝙩𝙞𝙤𝙣 𝙤𝙛 𝙎𝘼𝙍𝙎-𝘾𝙤𝙑-2 ?
It refers to the process by which the virus is targeted to lysosomes and broken down through the cell's lysosomal machinery.
And finally, 𝘼𝙪𝙩𝙤𝙥𝙝𝙖𝙜𝙮 𝙞𝙣𝙙𝙪𝙘𝙩𝙞𝙤𝙣 refers to ...
It refers to the process by which the virus is targeted to lysosomes and broken down through the cell's lysosomal machinery.
And finally, 𝘼𝙪𝙩𝙤𝙥𝙝𝙖𝙜𝙮 𝙞𝙣𝙙𝙪𝙘𝙩𝙞𝙤𝙣 refers to ...
7) ... the activation of this autophagy process in response to the infection caused by the SARS-CoV-2 virus.
After this short presentation, we can now present this study
"SARS-CoV-2 Omicron Envelope T9I adaptation confers resistance to autophagy"
biorxiv.org/content/10.110…

After this short presentation, we can now present this study
"SARS-CoV-2 Omicron Envelope T9I adaptation confers resistance to autophagy"
biorxiv.org/content/10.110…

8) The Omicron variant of SARS-CoV-2 is more resistant to autophagy induction compared to early 2020 SARS-CoV-2 strains and the Delta variant. Activation of autophagy reduced replication of early strains and Delta more strongly. 

9) Mutation T9I in the SARS-CoV-2 envelope (E) protein conferred increased autophagy resistance to Omicron variants compared to strains without this mutation. 

10) E mutation T9I leads to increased accumulation of autophagosomes by more strongly inhibiting autophagic flux. It interacts more with components of the autophagosome assembly machinery. 

11) Viral particles containing E T9I were less sensitive to autophagy induction upon entry compared to particles with E T9. However, E T9I did not alter particle assembly or infectivity. 

12) Rare Omicron isolates retaining the ancestral E T9 were more sensitive to autophagy induction compared to E T9I isolates. Recombinant early SARS-CoV-2 gained autophagy resistance by acquiring E T9I. 

13) In summary, the study identifies E T9I as a mutation that allows Omicron to escape autophagy, which may have contributed to its emergence and spread by evading this innate immune defense. Acquiring resistance to autophagy is an evolutionary adaptation of SARS-CoV-2. 

14) 𝙒𝙝𝙮 𝙩𝙝𝙞𝙨 𝙨𝙩𝙪𝙙𝙮 𝙞𝙨 𝙨𝙤 𝙞𝙢𝙥𝙤𝙧𝙩𝙖𝙣𝙩 ?
In several threads, we indicated that this E:T9I mutation by reducing the pathogenicity of Omicrons had changed the face of the pandemic.
There was a sort of trade-off among the Omicrons ...
In several threads, we indicated that this E:T9I mutation by reducing the pathogenicity of Omicrons had changed the face of the pandemic.
There was a sort of trade-off among the Omicrons ...
https://twitter.com/ejustin46/status/1705595823587758560?t=RXlizxutc65MIb-KTNXyJQ&s=19
15) ... less pathogenic but more infectious with better immune escape thanks to Spike mutations.
Omicron was therefore more dangerous, which is the case for a more infectious and less pathogenic virus.
Omicron was therefore more dangerous, which is the case for a more infectious and less pathogenic virus.

16) As we discovered that E:T9I may have contributed to the emergence and spread of Omicrons by evading the innate immune defense, it is really a key evolutionary adaptation of SARS-CoV-2.
Thanks for reading 🙏
H/t @DavidJoffe64 @siamosolocani @C_A_G0101
@mrmickme @DrInfoSec
Thanks for reading 🙏
H/t @DavidJoffe64 @siamosolocani @C_A_G0101
@mrmickme @DrInfoSec
• • •
Missing some Tweet in this thread? You can try to
force a refresh