𝗛5𝗡1 𝗟𝗔𝗦𝗧 𝗨𝗣𝗗𝗔𝗧𝗘 !
(🧵 𝘗𝘈𝘙𝘛 1)
"𝘗𝘳𝘦𝘭𝘪𝘮𝘪𝘯𝘢𝘳𝘺 𝘳𝘦𝘱𝘰𝘳𝘵 𝘰𝘯 𝘨𝘦𝘯𝘰𝘮𝘪𝘤 𝘦𝘱𝘪𝘥𝘦𝘮𝘪𝘰𝘭𝘰𝘨𝘺 𝘰𝘧 𝘵𝘩𝘦 2024 𝘏5𝘕1 𝘪𝘯𝘧𝘭𝘶𝘦𝘯𝘻𝘢 𝘈 𝘷𝘪𝘳𝘶𝘴 𝘰𝘶𝘵𝘣𝘳𝘦𝘢𝘬 𝘪𝘯 𝘜.𝘚. 𝘤𝘢𝘵𝘵𝘭𝘦 (𝘱𝘢𝘳𝘵 1)"
(🧵 𝘗𝘈𝘙𝘛 1)
"𝘗𝘳𝘦𝘭𝘪𝘮𝘪𝘯𝘢𝘳𝘺 𝘳𝘦𝘱𝘰𝘳𝘵 𝘰𝘯 𝘨𝘦𝘯𝘰𝘮𝘪𝘤 𝘦𝘱𝘪𝘥𝘦𝘮𝘪𝘰𝘭𝘰𝘨𝘺 𝘰𝘧 𝘵𝘩𝘦 2024 𝘏5𝘕1 𝘪𝘯𝘧𝘭𝘶𝘦𝘯𝘻𝘢 𝘈 𝘷𝘪𝘳𝘶𝘴 𝘰𝘶𝘵𝘣𝘳𝘦𝘢𝘬 𝘪𝘯 𝘜.𝘚. 𝘤𝘢𝘵𝘵𝘭𝘦 (𝘱𝘢𝘳𝘵 1)"

2) H/t @MichaelWorobey @PeacockFlu and colleagues
BACKGROUND :
Reassortment of the virus with local avian strains has generated diverse genotypes. virological.org/t/preliminary-…

BACKGROUND :
Reassortment of the virus with local avian strains has generated diverse genotypes. virological.org/t/preliminary-…

3) The virus has a high propensity to infect mammals, including domestic cats, foxes, mink, and pinnipeds. Mammalian infections lead to severe disease and quick adaptation. Bird-to-mammal transmission is uncertain. 

4) An outbreak of H5N1 was detected in dairy cattle in the USA, causing reduced milk production, severe mastitis, and mild respiratory disease. The full severity is unknown. 
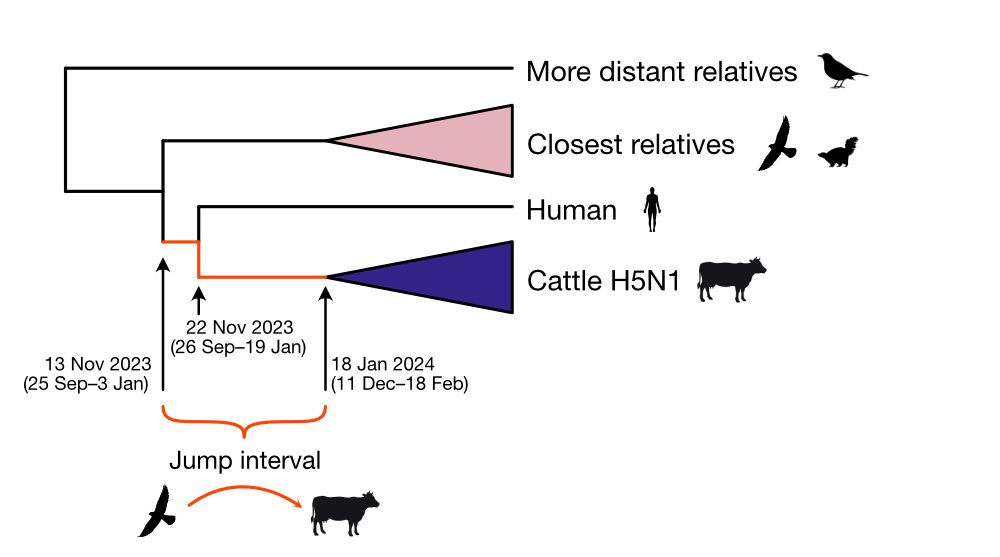
5) FINDINGS
A reassortment event occurred within the North American avian H5N1 2.3.4.4b viruses before the start of the cattle outbreak. The cattle sequences are all Genotype B3.13, which is a reassortant between the Eurasian panzootic H5N1 genotype and low pathogenicity ...
A reassortment event occurred within the North American avian H5N1 2.3.4.4b viruses before the start of the cattle outbreak. The cattle sequences are all Genotype B3.13, which is a reassortant between the Eurasian panzootic H5N1 genotype and low pathogenicity ...
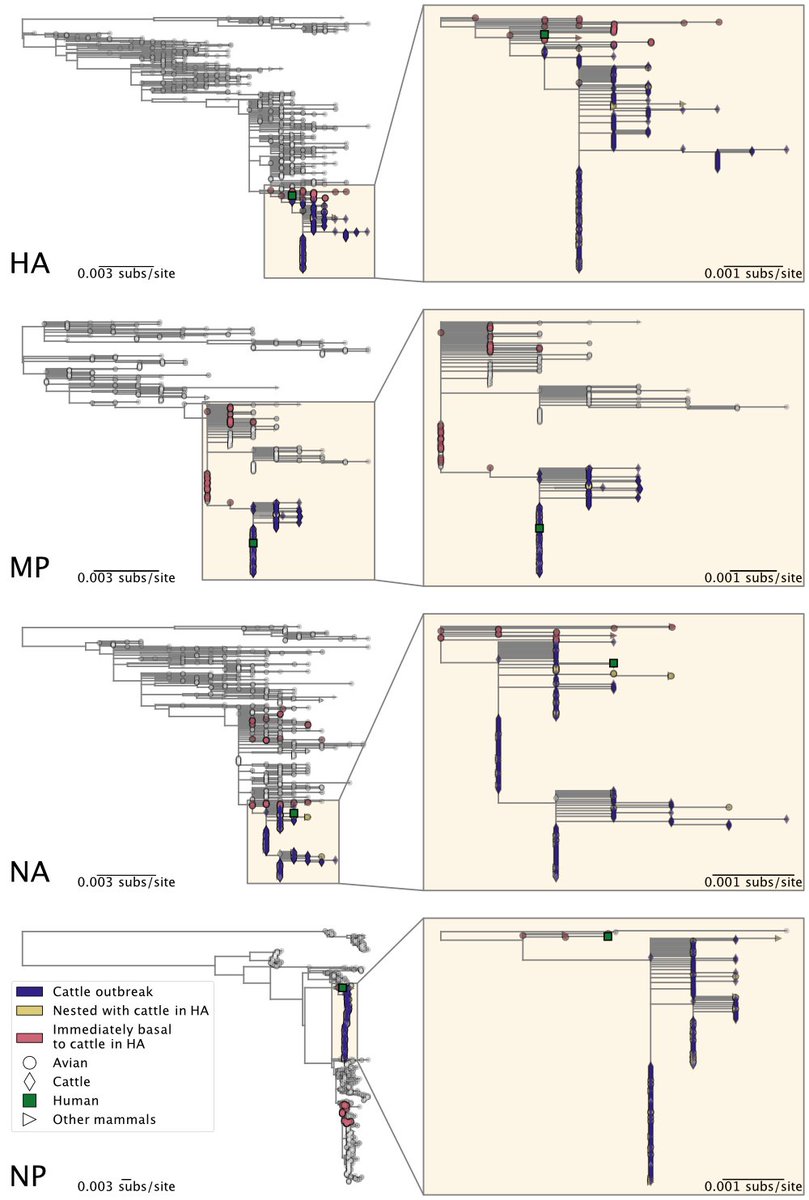
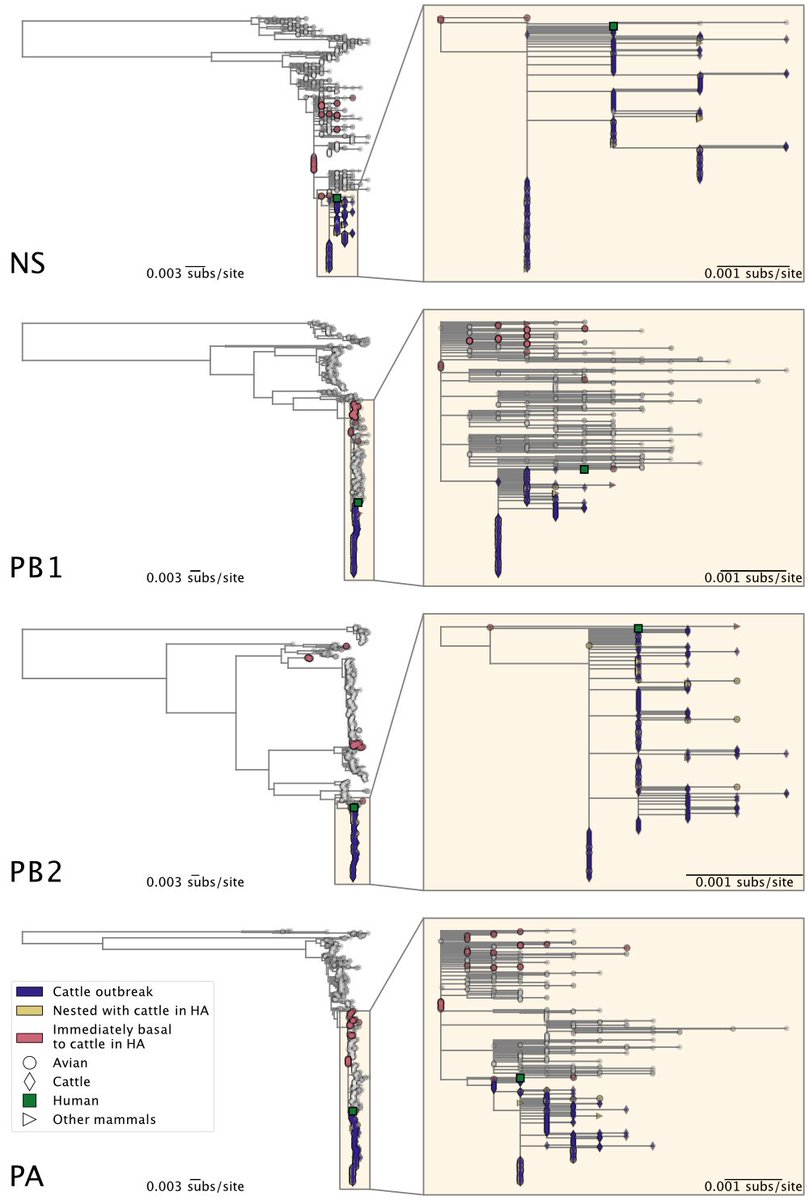
6) ... North American genotypes.
The cattle outbreak likely originated from a single introduction of H5N1 into cows and spread among cattle. The viruses sampled from cattle form a monophyletic clade in each genome segment, indicating cattle-to-cattle transmission.
The cattle outbreak likely originated from a single introduction of H5N1 into cows and spread among cattle. The viruses sampled from cattle form a monophyletic clade in each genome segment, indicating cattle-to-cattle transmission.
7) The H5N1 outbreak in cattle may have gone undetected and unidentified for an extended period of time. Molecular clock estimates suggest that the virus may have been circulating in cattle for up to 5 months before it was identified. 
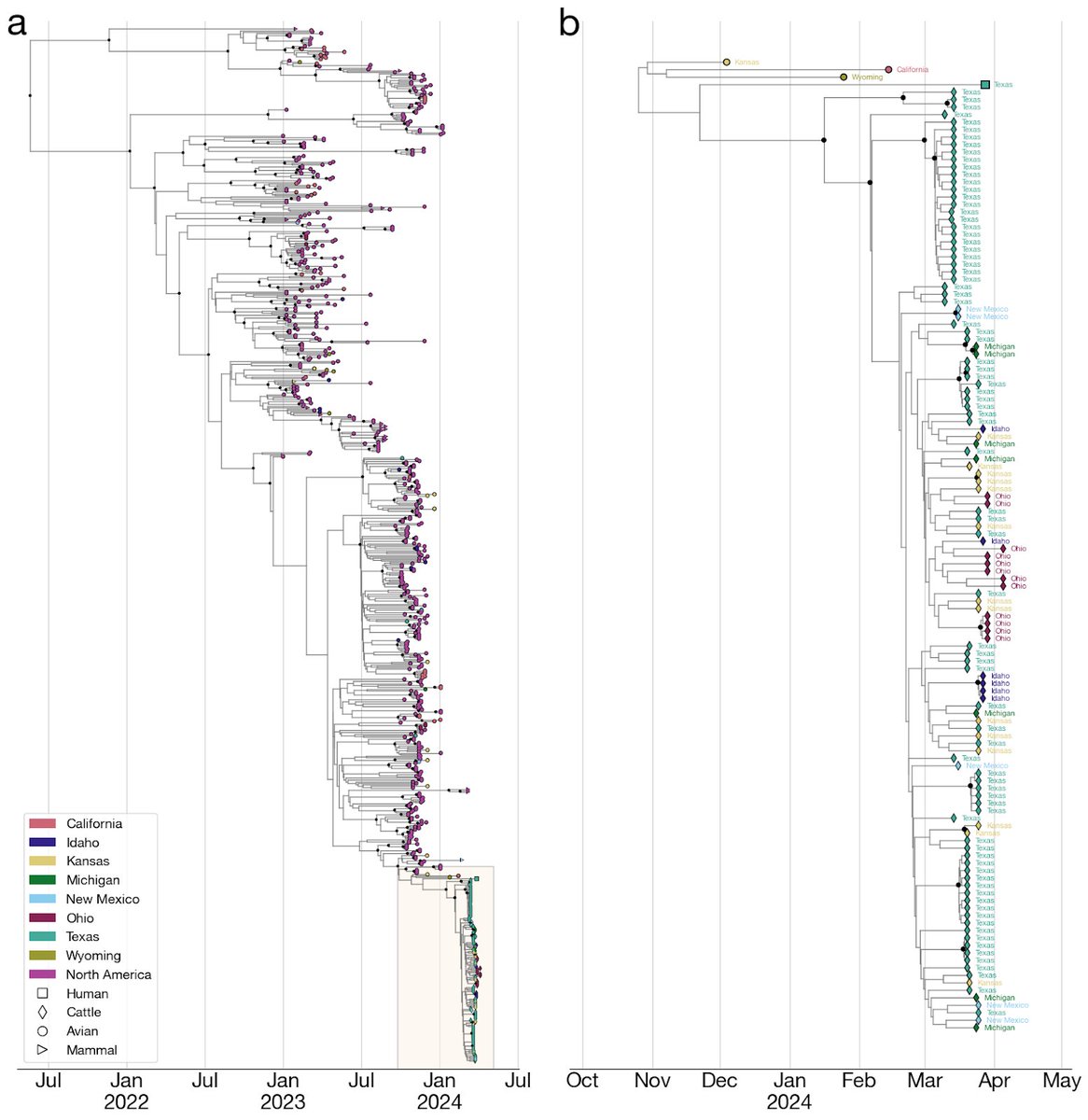
8) The cattle outbreak may have originated in Texas, where the first ill and infected cattle were reported. The phylogenetic tree shows basal diversity sampled in Texas, but further analysis is needed to determine the movement of the virus across states. 
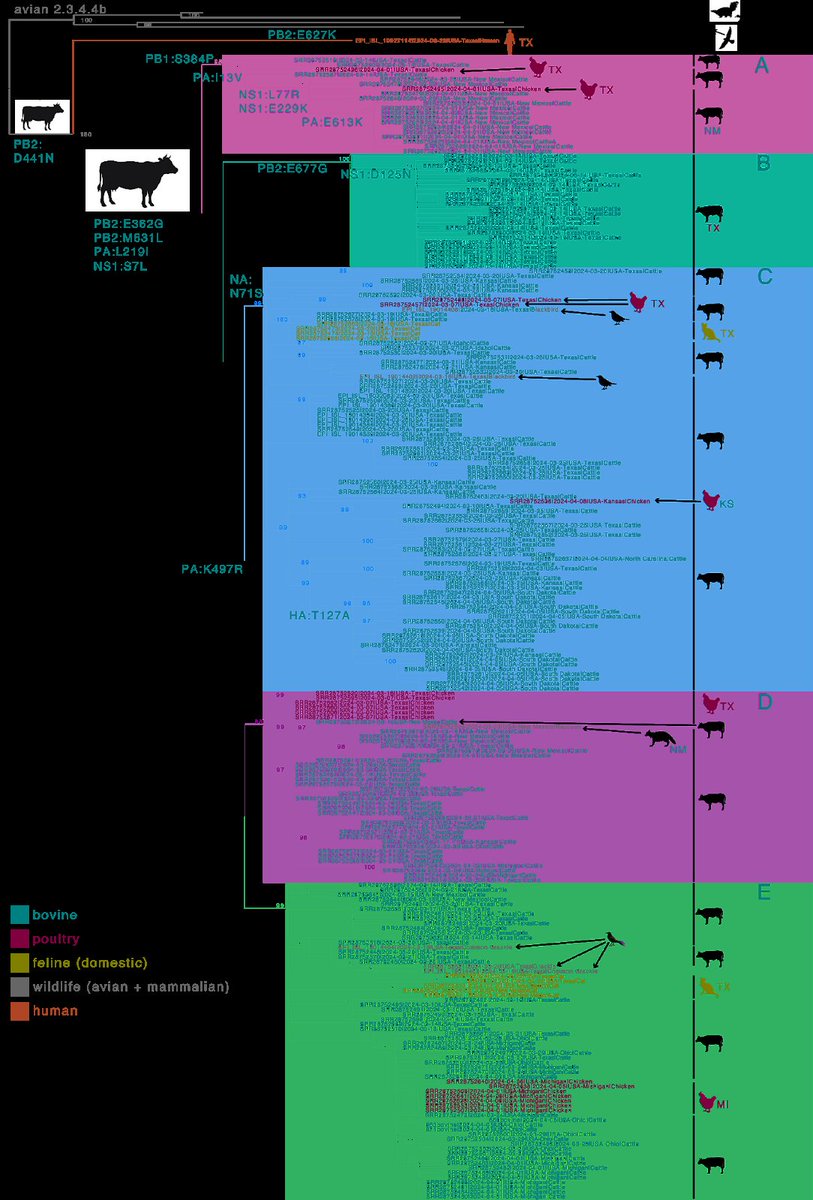
9) The cattle H5N1 clade has several putatively adaptive substitutions in the polymerase complex, which are necessary for the virus to infect mammals. These substitutions indicate adaptation to use mammalian versions of a host protein called ANP32.
10) There is minimal evidence for a different selective regime acting within the cattle H5N1 virus clade compared to avian H5N1 viruses, except for a modest increase in the intensity of selection in the PA segment.
Thanks for reading 🙏
2nd part in few minutes 🙋♂️
Thanks for reading 🙏
2nd part in few minutes 🙋♂️
• • •
Missing some Tweet in this thread? You can try to
force a refresh