𝙐𝙣𝙥𝙧𝙚𝙙𝙞𝙘𝙩𝙖𝙗𝙞𝙡𝙞𝙩𝙮 𝙤𝙛 𝙫𝙞𝙧𝙖𝙡 𝙚𝙫𝙤𝙡𝙪𝙩𝙞𝙤𝙣 𝙤𝙛 𝙎𝘼𝙍𝙎-𝘾𝙊𝙑-2
𝘒𝘦𝘺 𝘴𝘵𝘶𝘥𝘺 𝘸𝘪𝘵𝘩 @MarionKoopmans @PeacockFlu @mvankerkhove
@firefoxx66 @world_epidemic
... 𝘢𝘯𝘥 𝘤𝘰𝘭𝘭𝘦𝘢𝘨𝘶𝘦𝘴
who.int/publications-d…

𝘒𝘦𝘺 𝘴𝘵𝘶𝘥𝘺 𝘸𝘪𝘵𝘩 @MarionKoopmans @PeacockFlu @mvankerkhove
@firefoxx66 @world_epidemic
... 𝘢𝘯𝘥 𝘤𝘰𝘭𝘭𝘦𝘢𝘨𝘶𝘦𝘴
who.int/publications-d…
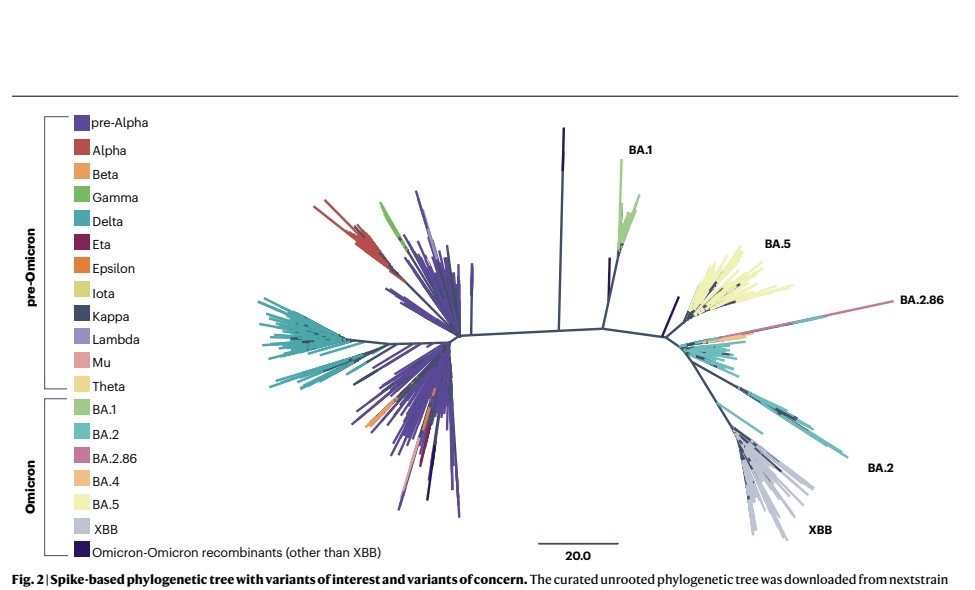
2) The WHO has updated their framework for classifying SARS-CoV-2 variants of concern and variants of interest to better reflect the evolution of the virus.
Omicron and its many descendant lineages have become globally dominant and presented new challenges for classification.
Omicron and its many descendant lineages have become globally dominant and presented new challenges for classification.
3) Under the updated system, Omicron descendants can be independently evaluated as VOCs, VOIs or variants under monitoring (VUMs).
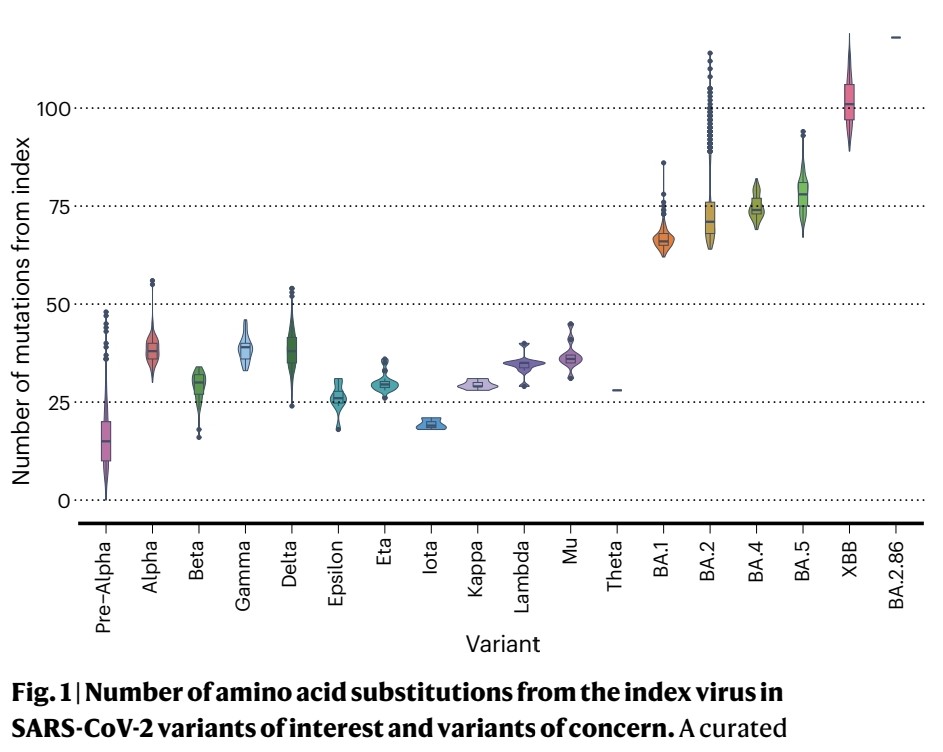
XBB and its descendent lineages have spread globally in 2022-2023 due to an immune escape advantage.
XBB and its descendent lineages have spread globally in 2022-2023 due to an immune escape advantage.
4) XBB.1 in particular reduced antibody titers from index virus vaccines.
The impact of new variants is lessening as population immunity increases from vaccination and infection. Severe disease is now prioritized over transmission advantage in risk assessments.
The impact of new variants is lessening as population immunity increases from vaccination and infection. Severe disease is now prioritized over transmission advantage in risk assessments.
5) Continued genomic surveillance is important to rapidly identify properties of new variants that could evade immunity or change disease severity/tropism profiles. Animal surveillance also provides insight into viral evolution pathways.
6) Thanks for reading 🙏 and a great great thanks, to a fantastic australian physician and great friend @DavidJoffe64 who sent me this study 😊
• • •
Missing some Tweet in this thread? You can try to
force a refresh