An interesting re-analysis was published today: "Remdesivir treatment does not reduce viral titers in patients with COVID-19"
Basically, remdesivir has no impact on *viral load* in acute COVID!
Here's a summary of the findings—and controversy—for a general audience!
1/12
Basically, remdesivir has no impact on *viral load* in acute COVID!
Here's a summary of the findings—and controversy—for a general audience!
1/12

Initially, remdesivir received emergency FDA approval because in one NIH-sponsored trial, the remdesivir group recovered quicker than the control group. That's ALL.
It was trialed because it does seem to be a great drug in cell cultures in the lab!
2/


It was trialed because it does seem to be a great drug in cell cultures in the lab!
2/


It SEEMED like it would be a great drug, because it does exactly what we want *in cell cultures*. Unfortunately, even in animal models, it seemingly had issues.
In particular, viral load was lower in fluid from the lungs, but not in nose, throat, or rectal swabs.
3/
In particular, viral load was lower in fluid from the lungs, but not in nose, throat, or rectal swabs.
3/

In humans, no study has shown a statistically significant decrease in viral load as a result of remdesivir treatment!
Only two studies looked at changes in viral load under remdesivir treatment and both found NO significant differences compared to controls.
4/!["In humans, RDV has failed to demonstrate statistically significant reductions in viral titers compared to placebo control groups. [...] However, only two studies have documented the changes in viral titers associated with RDV treatment: (i) Wang et al. Lancet (2020), who published the first double-blind RCT of remdesivir versus placebo-treated patients hospitalized with severe COVID-19 and (ii) Killingley et al. Nature Medicine (2022), who published the results of the SARS-CoV-2 challenge study in healthy human volunteers. In the study by Wang and colleagues, viral titers were measur...](/images/1px.png)
Only two studies looked at changes in viral load under remdesivir treatment and both found NO significant differences compared to controls.
4/
!["In humans, RDV has failed to demonstrate statistically significant reductions in viral titers compared to placebo control groups. [...] However, only two studies have documented the changes in viral titers associated with RDV treatment: (i) Wang et al. Lancet (2020), who published the first double-blind RCT of remdesivir versus placebo-treated patients hospitalized with severe COVID-19 and (ii) Killingley et al. Nature Medicine (2022), who published the results of the SARS-CoV-2 challenge study in healthy human volunteers. In the study by Wang and colleagues, viral titers were measur...](https://pbs.twimg.com/media/GS0i5ktXwAAF_Wf.jpg)
Apparently, one of the studies is criticized for having a small sample size... but that makes it much more likely that they'll have a false POSITIVE result, especially when it seemed to be so effective in preclinical research!
5/
5/

In a challenge trial in 2022 (dear researchers: NO SC2 CHALLENGE TRIALS, WTF?), they provided remdesivir as a prophylactic against severe COVID, but the data suggested it might not be needed.
Thus, they discontinued administration for the rest of the participants.
6/
Thus, they discontinued administration for the rest of the participants.
6/

This change in protocol created a quasi-experiment, which is the focus here!
So, even in a study where participants were exposed to a standardized quantity of virus, no differences in viral load were found between the treated-with-remdesivir group and the no-remdesivir group.
7/
So, even in a study where participants were exposed to a standardized quantity of virus, no differences in viral load were found between the treated-with-remdesivir group and the no-remdesivir group.
7/

Boiling it down:
- It shows a certain level of viral inhibition in the lab
- Some studies have found it may have an impact on OUTCOMES
- But it DOESN'T have an impact on the viral load
The issue seems to be that the dose in the body can't get high enough!
8/
- It shows a certain level of viral inhibition in the lab
- Some studies have found it may have an impact on OUTCOMES
- But it DOESN'T have an impact on the viral load
The issue seems to be that the dose in the body can't get high enough!
8/

HOWEVER! Meta-analyses have noted SOME sort of SMALL impact on mortality, for SOME patients, e.g.:
-
-
-
Maybe especially immunocompromised:
-
So what's up?
9/pubmed.ncbi.nlm.nih.gov/35598856/
pubmed.ncbi.nlm.nih.gov/36828006/
pubmed.ncbi.nlm.nih.gov/35512728/
pubmed.ncbi.nlm.nih.gov/38105461/
-
-
-
Maybe especially immunocompromised:
-
So what's up?
9/pubmed.ncbi.nlm.nih.gov/35598856/
pubmed.ncbi.nlm.nih.gov/36828006/
pubmed.ncbi.nlm.nih.gov/35512728/
pubmed.ncbi.nlm.nih.gov/38105461/
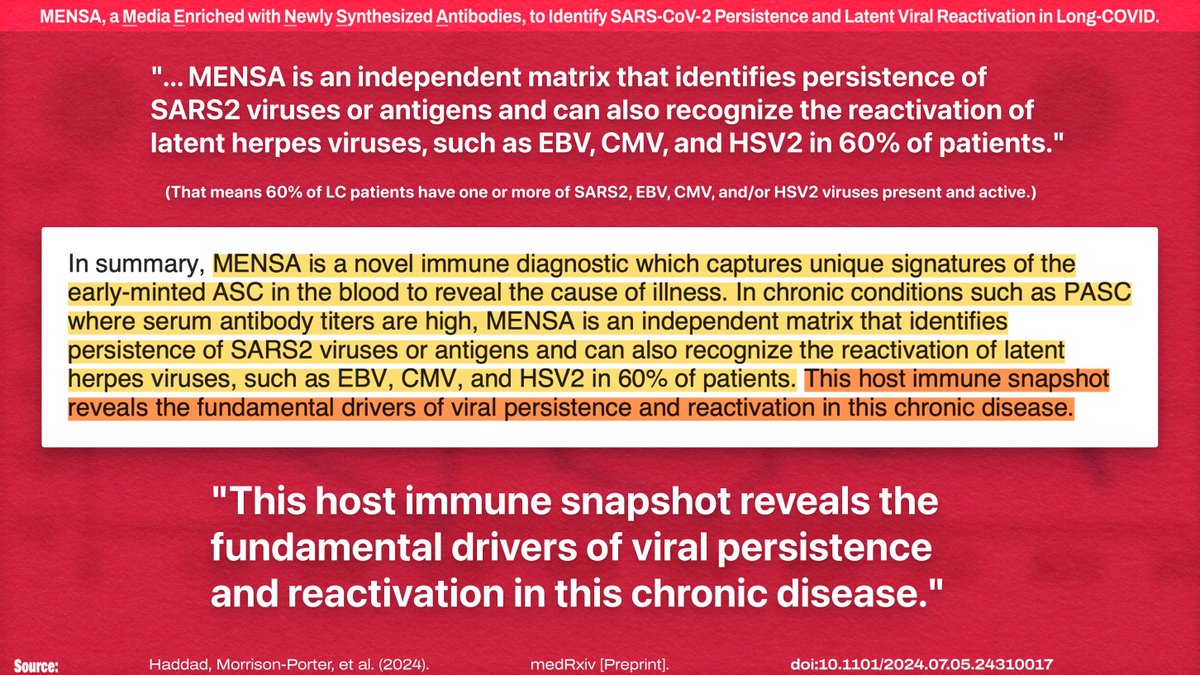
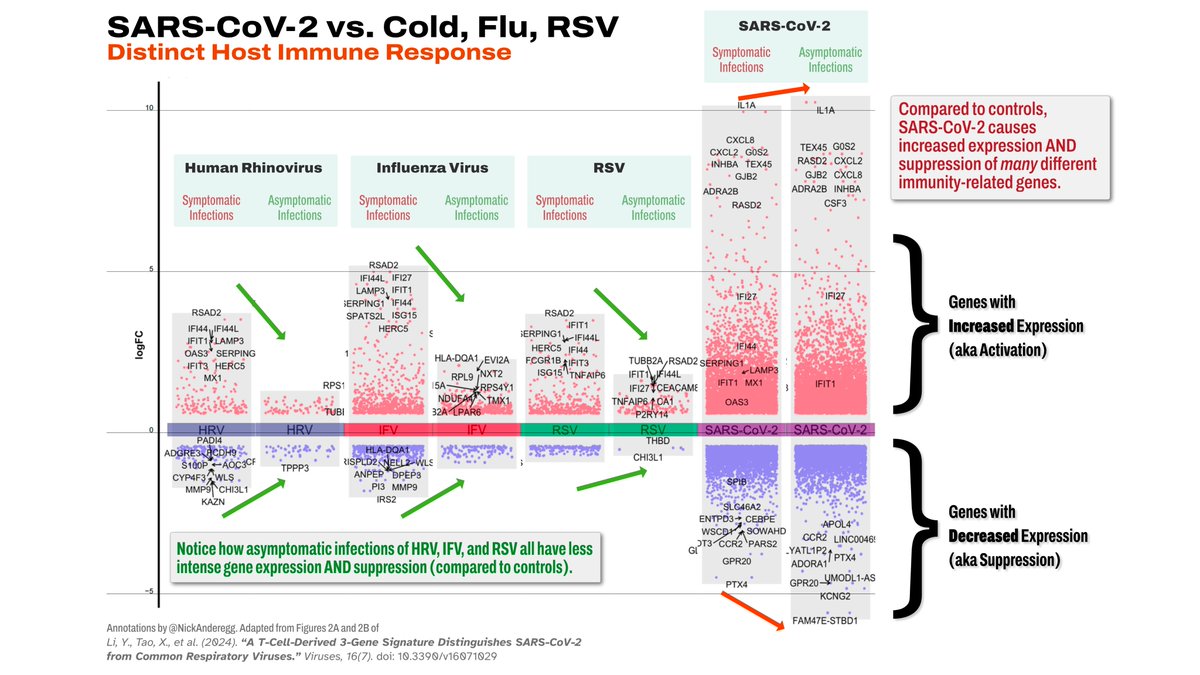
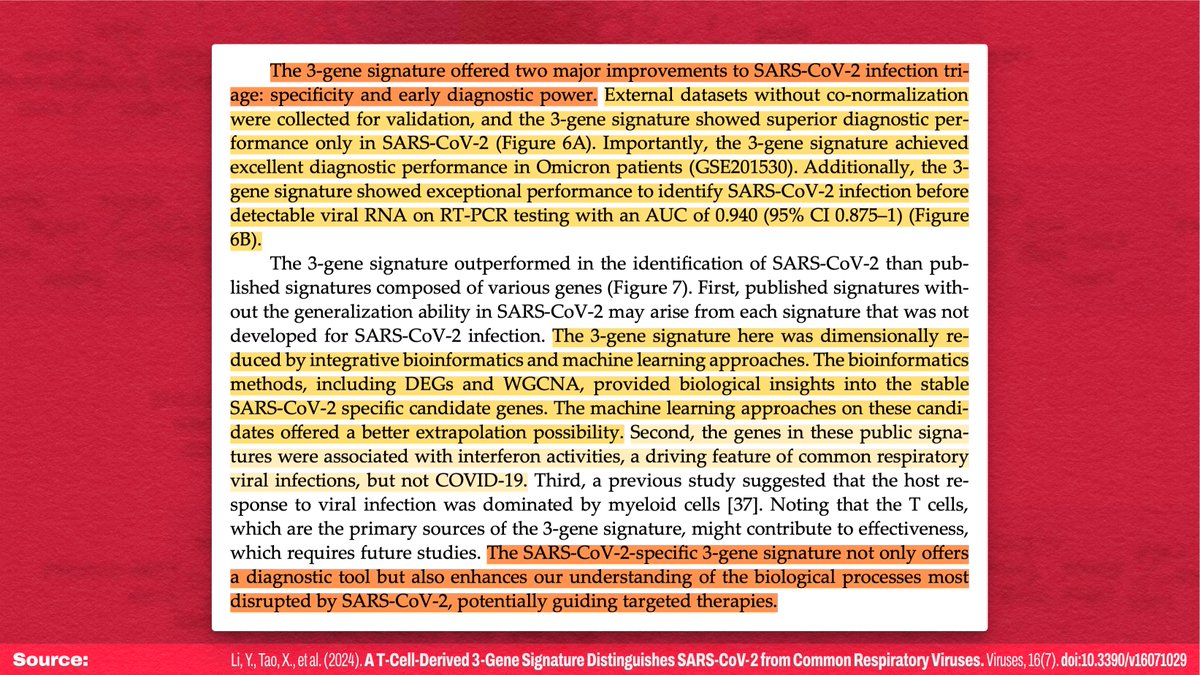
IMO, I think the most likely answer is that some sort of latent viral reactivation is playing a role here (e.g.: ).
Remdesivir does seem to be a good antiviral in vitro, so if it has an impact on some other virus, it could skew the clinical outcomes!
10/
Remdesivir does seem to be a good antiviral in vitro, so if it has an impact on some other virus, it could skew the clinical outcomes!
10/
https://x.com/NickAnderegg/status/1810137709677105552
That means a lot more research is needed on the specific factors that lead to severe acute outcomes (and LC!), allowing specific diagnostics, allowing specific treatments.
No clinical trial can be successful if unidentified underlying issues aren't being addressed!
11/
No clinical trial can be successful if unidentified underlying issues aren't being addressed!
11/
For the record, this is very different from my first draft of this thread.
After I realized it was written by *current* grad/med students, I rewrote it as an actual critique, instead of my usual incisive schtick
Paper:
12/12journals.asm.org/doi/10.1128/aa…
After I realized it was written by *current* grad/med students, I rewrote it as an actual critique, instead of my usual incisive schtick
Paper:
12/12journals.asm.org/doi/10.1128/aa…
• • •
Missing some Tweet in this thread? You can try to
force a refresh


![Admission screening testing of patients and staff N95 masks are cost-effective in reducing COVID-19 hospital acquired infections "Findings: Compared to no admission screening testing and staff surgical masks, all scenarios were cost saving with health gains. Staff N95s + RAT admission screening of patients was the cheapest, saving A$78.4M [95%UI 44.4M-135.3M] and preventing 1,543 [1,070-2,146] deaths state-wide per annum. Both interventions were individually beneficial: staff N95s in isolation saved A$54.7M and 854 deaths state-wide per annum, while RAT admission screening of patients...](https://pbs.twimg.com/media/GSvUAAaXAAAaNv9.jpg)
!["... Hospital-acquired COVID-19 infections occur when patients admitted for non-COVID-19 reasons acquire COVID-19 following exposure to staff, other patients or visitors, and infection prevention and control measures are insufficient to prevent transmission. As well as being a patient safety risk, hospital-acquired infections carry significant costs to the health system [2-4]. [...] In the state of Victoria, 15-25% of patients in hospital with COVID-19 between June 2022-June 2023 acquired their infection after admission, with 90-day mortality of 18.9% compared to 12.3% among matched pa...](https://pbs.twimg.com/media/GSvUCjQWAAAsTSS.jpg)


!["What information was available about SARS in the scientific literature before the COVID-19 pandemic? Laboratory practices and medical treatment guidelines were published during and shortly after the 2003 SARS outbreak, regarding coronavirus identification by RT-PCR, epidemiology, and containment strategies. Clinical chemistry guidelines [22], diagnosis based on RT-PCR [23-26] and indications for therapeutic treatment were readily available from the experience with SARS. Although this knowledge was available well before the COVID-19 pandemic, much of it was "rediscovered" in...](https://pbs.twimg.com/media/GSfbDwHXwAQKpX_.jpg)
!["Similarly, the use of hyper-immune serum from recovered patients was indicated among the successful therapeutic interventions during the SARS outbreak [29] and was apparently rediscovered, as a new and innovative strategy, during the COVID-19 pandemic. Also the increased incidence of Kawasaki-like disease (or rather "syndrome" as this clinical presentation of the SARS-CoV-2 infection in children is now called), had been described during the SARS outbreak, and has occurred again during the COVID-19 pandemic [30], as should have been expected, given the much larger prevalence ...](https://pbs.twimg.com/media/GSfbFL9WUAUiG0r.jpg)



!["During the SARS-CoV-2 pandemic, some authors suggested the potential usefulness of IMQ as an immunostimulant to enhance individuals' immune readiness before exposure to the virus. However, there are no reported clinical studies or suggested application protocols for this specific purpose so far. Imiquimod (1-isobutyl-1H-imidazo [4,5-c]-quinolin-4-amine(C14H16N4)) is a synthetic imidazoquinoline with a molecular weight of 240.31 g•mol-1."](https://pbs.twimg.com/media/GSVXYojWoAArNJn.jpg)
![Preprint Title: "MENSA, a Media Enriched with Newly Synthesized Antibodies, to Identify SARS-CoV-2 Persistence and Latent Viral Reactivation in Long-COVID." Abstract: "Here, we use MENSA, Media Enriched with Newly Synthesized Antibodies, secreted exclusively from circulating human plasmablasts, to provide an immune snapshot that defines the underlying viral triggers. [...] Applying the same principles for long-COVID patients, MENSA is positive for SARS2 in 40% of PASC vs none of the COVID recovered (CR) patients without any sequelae demonstrating ongoing SARS2 viral inflamma...](https://pbs.twimg.com/media/GR7lGksW8AEcoDF.jpg)