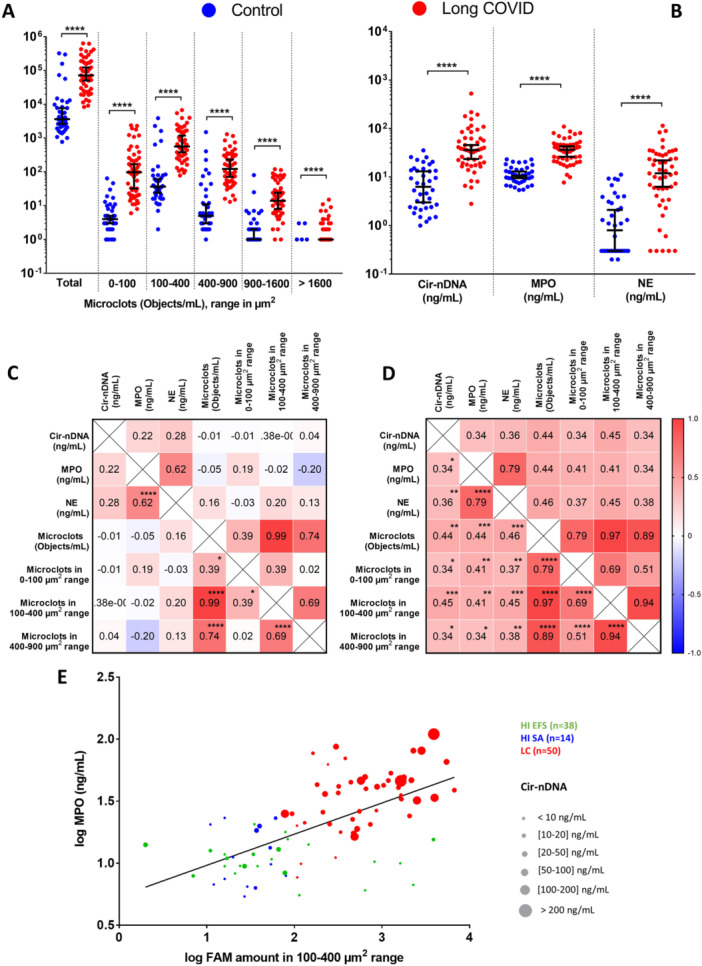
IMMUNITY CANNOT BE EXPLAINED by NEUTRALIZING ANTIBODIES (nAbs) ALONE !
Great thanks to @DavidJoffe64
for this study
nature.com/articles/s4159…

Great thanks to @DavidJoffe64
for this study
nature.com/articles/s4159…

2) The study looked at what immune responses protect people from getting infected with different variants of the COVID-19 virus. It focused on antibodies that can neutralize the virus (called neutralizing antibodies or nAbs). 

3) For the Delta variant, the researchers found that nAbs against the original COVID-19 strain (called D614G) accounted for 37% of the protection people had from prior infection. But this protection decreased over time as the antibodies weakened. 

4) For the Omicron variant, nAbs against the Omicron BA.1 subvariant only accounted for 11% of the protection. This is because Omicron is much better at evading antibodies compared to earlier variants. 

5) The study shows that the immune responses that protect against different COVID-19 variants can be very different. Boosting antibodies against the current variants may help restore protection that is lost over time or due to immune evasion by new variants. 

6) However, antibodies alone don't fully explain the protection people have. Other immune responses, like those from immune cells, are also likely important for preventing COVID-19 infection.
Thanks for reading 🙏
Thanks for reading 🙏

• • •
Missing some Tweet in this thread? You can try to
force a refresh