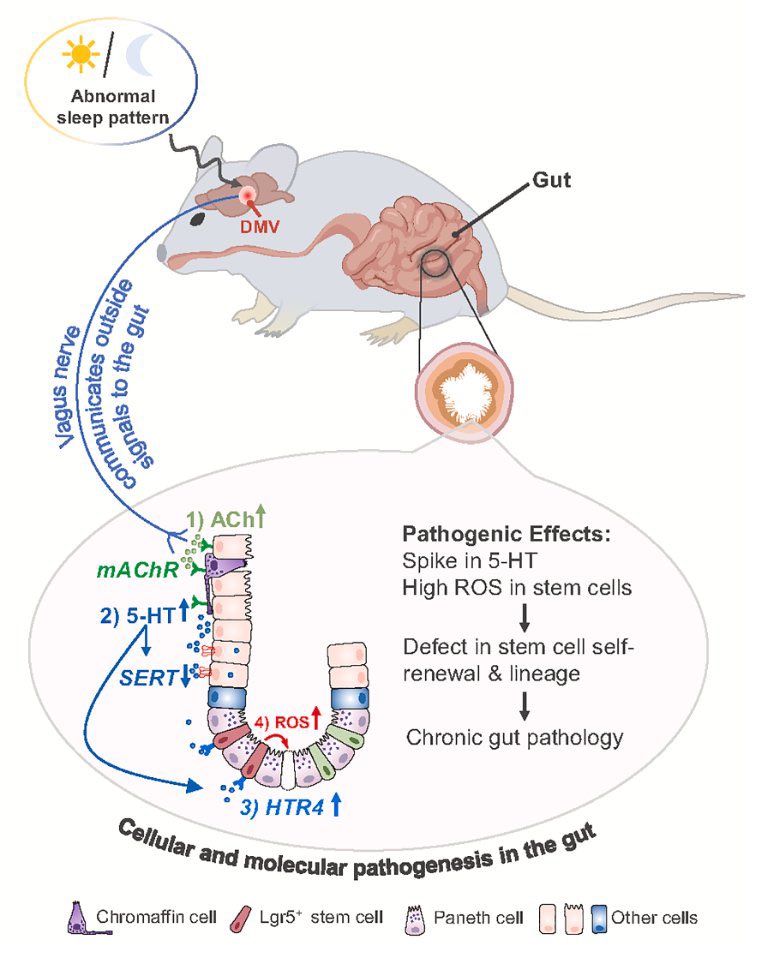
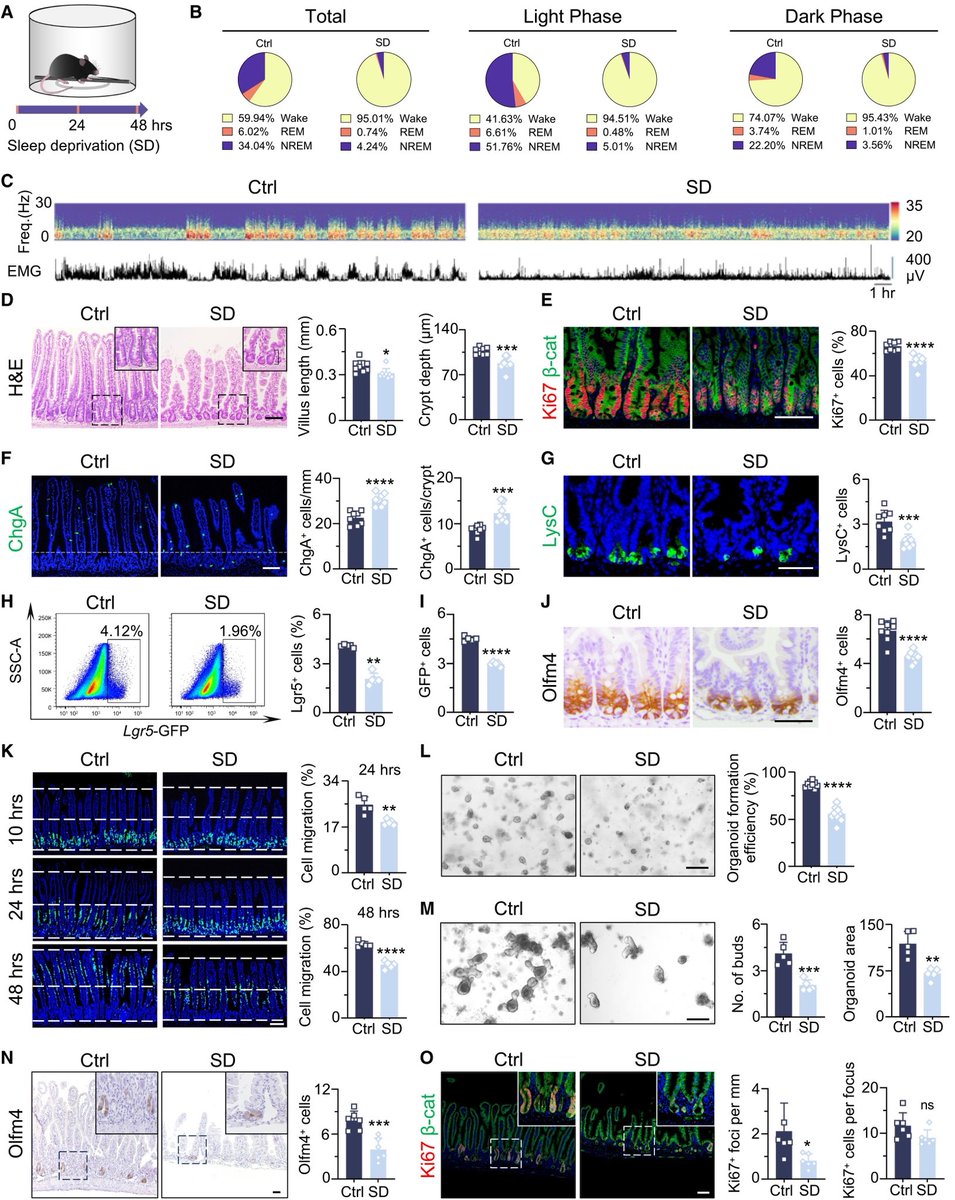
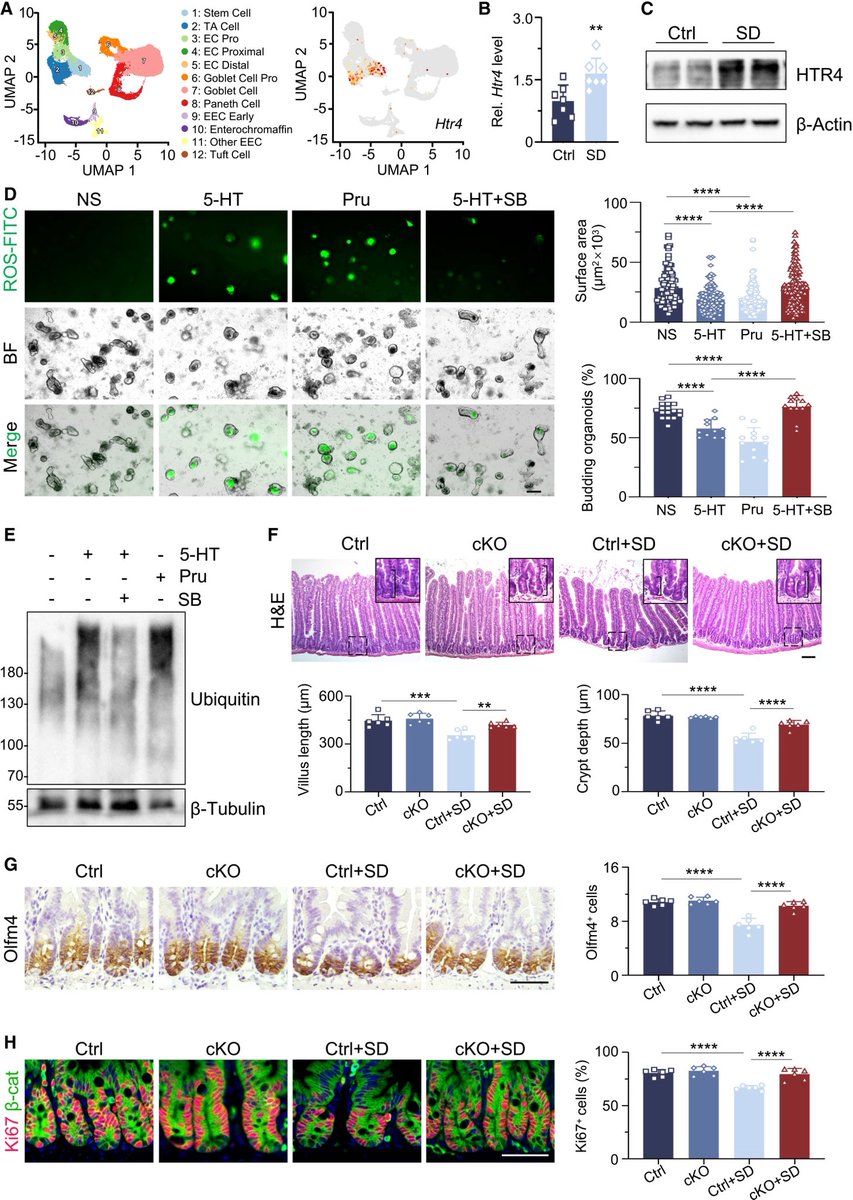
A novel treatment for blocking SARS-CoV-2 entry into cells!
Researchers discovered #UNI418, a compound that effectively prevents the penetration of coronavirus. This compound works by regulating dielectric homeostasis, thereby inhibiting the virus's entry into human cells 1/
Researchers discovered #UNI418, a compound that effectively prevents the penetration of coronavirus. This compound works by regulating dielectric homeostasis, thereby inhibiting the virus's entry into human cells 1/

SARS-CoV-2 enters cells through endocytosis, a process whereby cells absorb material from outside by engulfing it w/ their cell membrane. They demonstrated that inhibiting specific proteins, PIKfyve & PIP5K1C during this process can prevent viral invasion. 2/ 

Genomic homeostasis is protective system that secures genetic information & allows it to be utilized when needed.
The team established #UNI418 supports genomic homeostasis while simultaneously preventing the infiltration and proliferation of coronaviruses within cells 3/


The team established #UNI418 supports genomic homeostasis while simultaneously preventing the infiltration and proliferation of coronaviruses within cells 3/


Existing treatments generally work by inhibiting viral proteins to prevent proliferation, but they are often less effective against mutant strains of the virus. 4/ 

This study represents the first evidence that #UNI418 can disrupt the virus's infection process, highlighting its potential as a treatment for mutant coronaviruses and other viral infections. 5/ 

There is a high likelihood that UNI418 can develop into a new treatment paradigm that effectively blocks various viral infections. 6/6
nature.com/articles/s1227…

nature.com/articles/s1227…

• • •
Missing some Tweet in this thread? You can try to
force a refresh