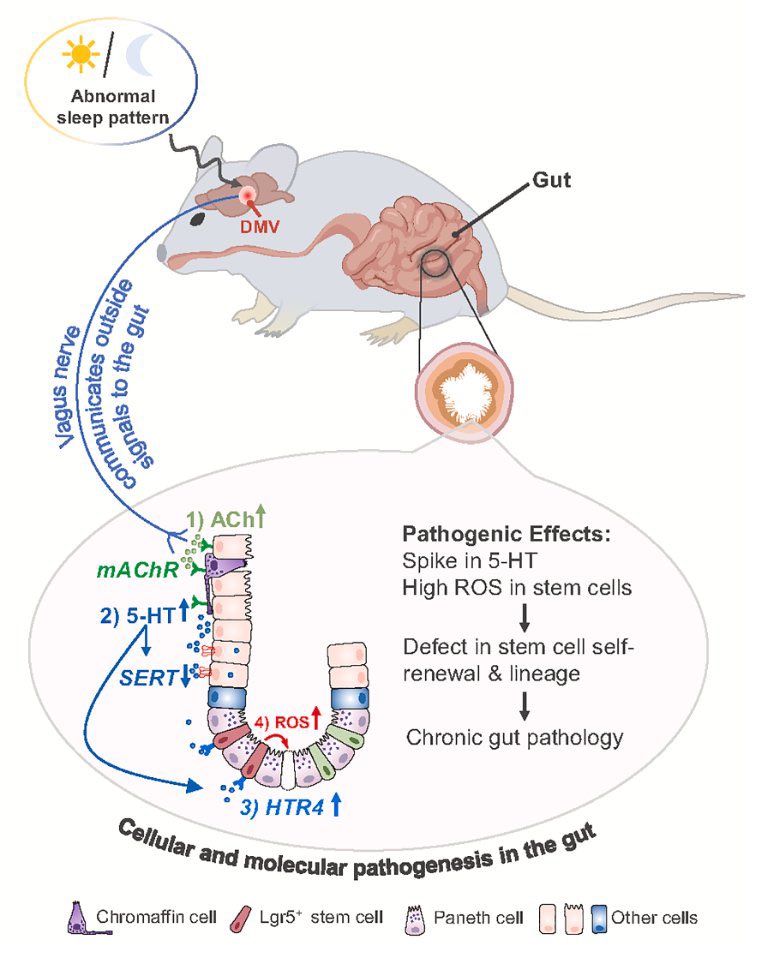
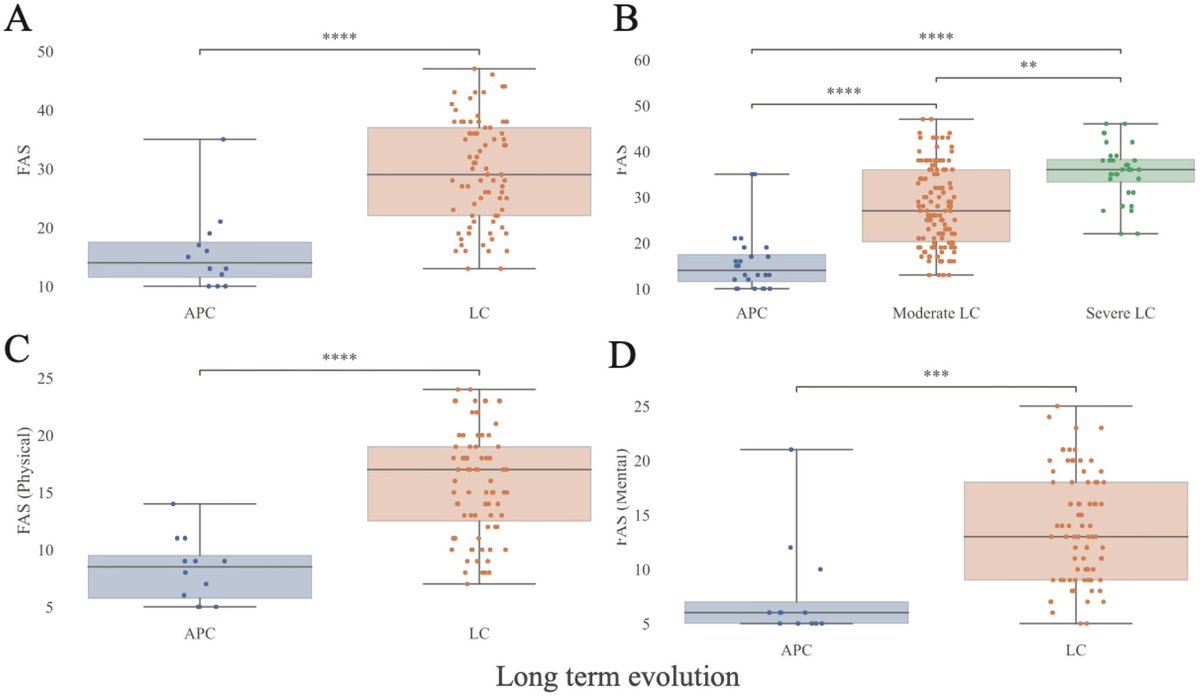
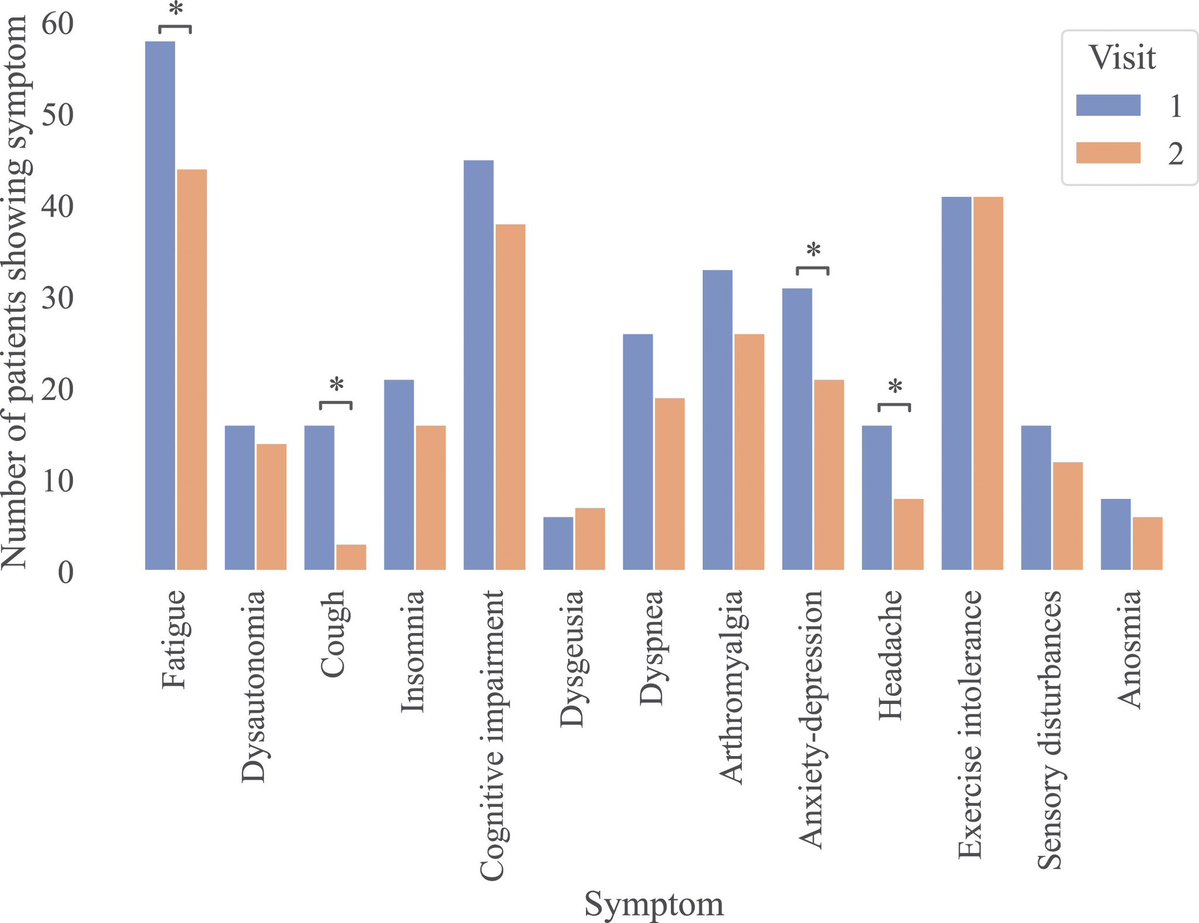
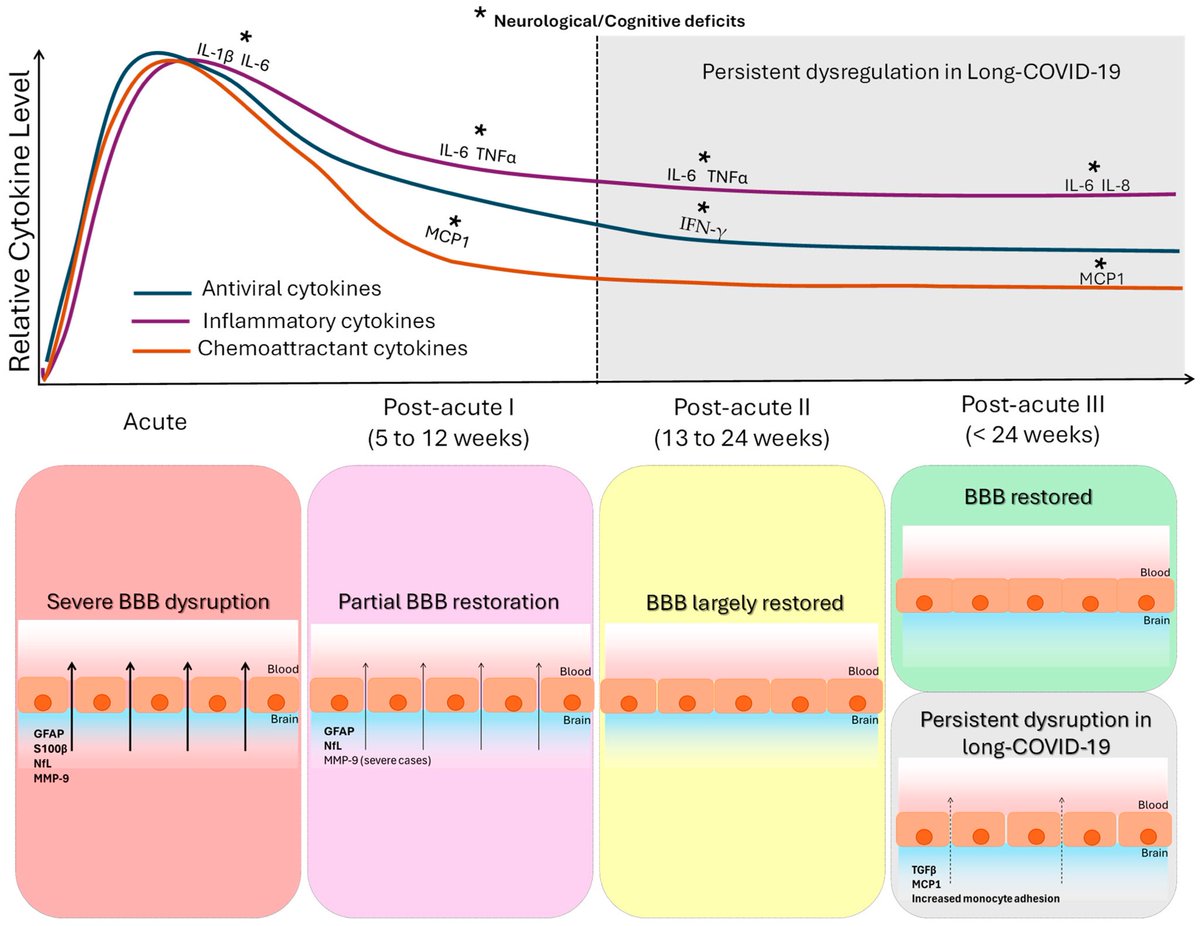
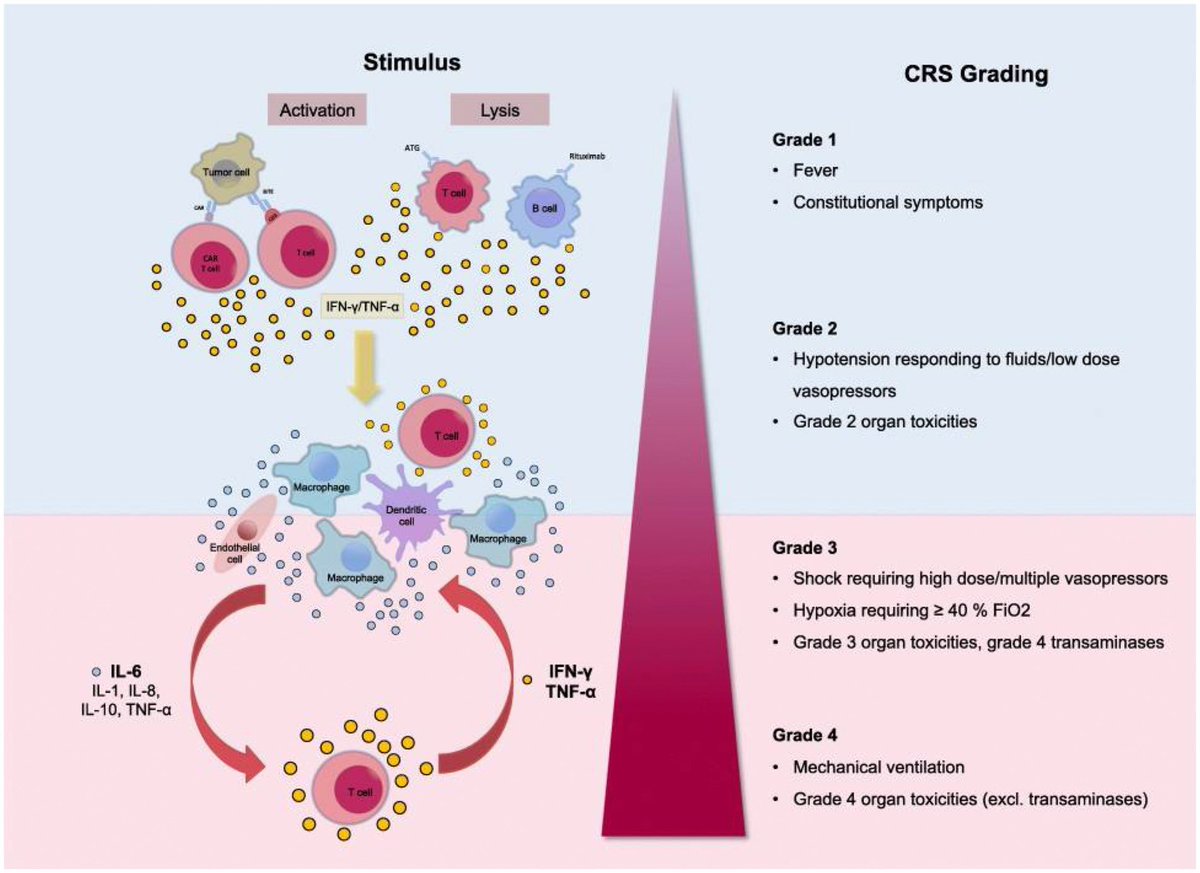
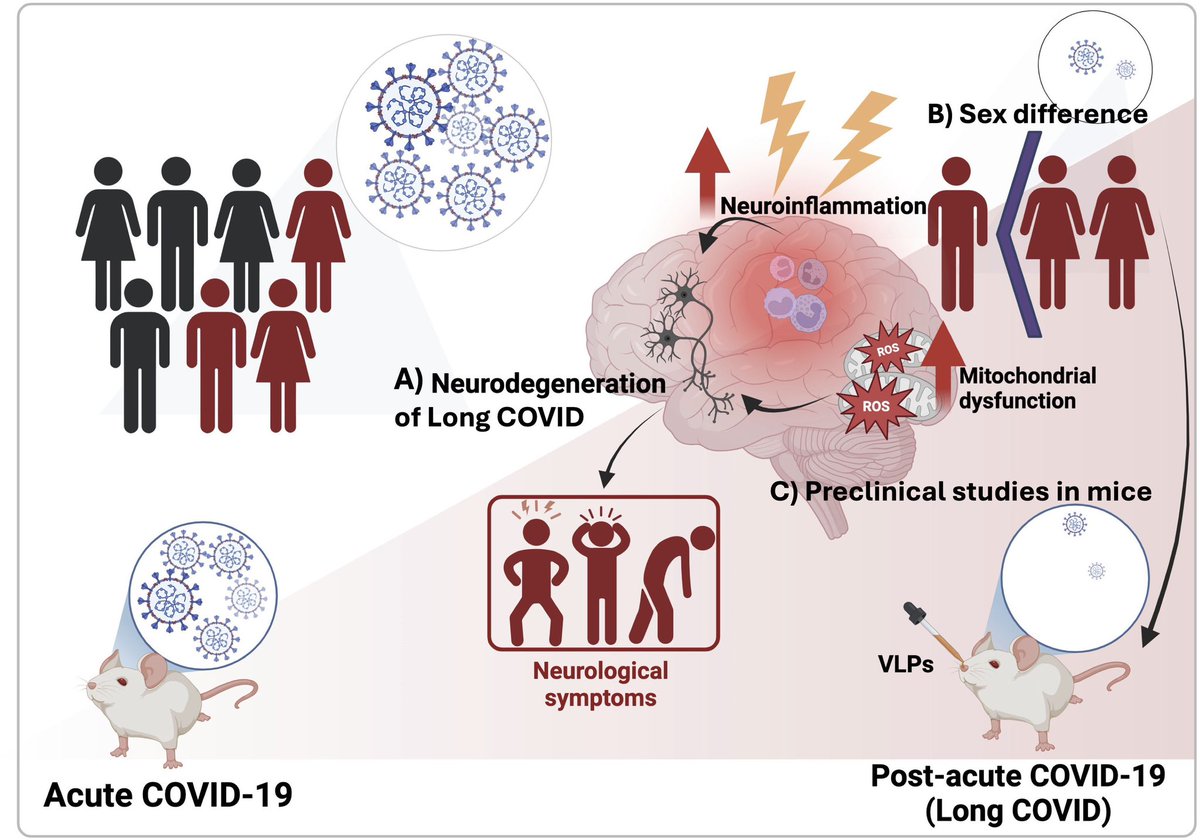
What triggers neuroinflammation?
Why is our brain so prone to inflammation?
🔥 A recent pathbreaking study explains the molecular mechanisms that trigger neuroinflammation. It shows exactly why neuroinflammation & neurodegeneration are so common. 1/
Why is our brain so prone to inflammation?
🔥 A recent pathbreaking study explains the molecular mechanisms that trigger neuroinflammation. It shows exactly why neuroinflammation & neurodegeneration are so common. 1/

But before we delve into this new discovery, let’s glance over a new study linking SARS-CoV-2 & later development of neurodegenerative brain disorders by inducing amyloid aggregation of proteins in human CSF & result in soluble protein depletion. 2/
biorxiv.org/content/10.110…

biorxiv.org/content/10.110…

Now coming back to this extraordinary research. We know that all living cells teem with RNA that relay genetic messages to keep cells functioning. But these necessary molecules can also set off cellular alarms. 3/ 

The long twisted-together strands of RNA in viruses, for instance, are a tell-tale sign of an invader and the human immune system triggers inflammation in their presence. 4/ 

Let's assume a X-virus carries double-stranded RNA (dsRNA) instructions. After the X virus deposited it’s RNA, the viral dsRNA is immediately detected by a family of sensors inside the cell called pattern recognition receptors (PRRs), like TLR3 5/
H/t: @BrainInflCollab
H/t: @BrainInflCollab

@BrainInflCollab TLR3 stimulation by viral dsRNA makes Interferons (IFNs), a pro-inflammatory proteins made and released by host cells in response to the presence of pathogens such as viruses, bacteria, parasites or tumor cells. 6/ 

@BrainInflCollab But how immune system could tell the difference between disease-causing viral RNA & our own normal RNA.
Now, that question led researchers in a surprising direction—the study of brain diseases including Alzheimer's, amyotrophic lateral sclerosis (ALS) & lupus brain fog 7/
Now, that question led researchers in a surprising direction—the study of brain diseases including Alzheimer's, amyotrophic lateral sclerosis (ALS) & lupus brain fog 7/

@BrainInflCollab The results suggest that the incorrect sensing of our own RNA in the brain may be to blame for chronic inflammation in conditions like these. 8/ 

@BrainInflCollab Scientists have long known that immune molecules responsible for recognizing viruses, known as pattern recognition receptors (PRRs), can detect viral double-stranded RNA structures, which form when two complementary RNA strands bond together. 9/
nature.com/articles/s4139…

nature.com/articles/s4139…

@BrainInflCollab The researchers initially studied if the RNA modifying enzyme ADAR1 could target viral dsRNAs and alter the course of viral infection.
*Here ADAR1 is the key.
What’s it & how it functions? 10/


*Here ADAR1 is the key.
What’s it & how it functions? 10/


@BrainInflCollab ADAR1 is an RNA-editing enzyme that performs a variety of functions, including editing dsRNA: converts adenosine to inosine. This process can disrupt dsRNA structures & modulate RNA metabolism. ADAR1 deficiency results in inflammatory diseases 11/
mdpi.com/2073-4425/7/12…

mdpi.com/2073-4425/7/12…

@BrainInflCollab Another researcher at the same time discovered that mutations disrupting the function of ADAR1 cause the autoimmune disease Aicardi-Goutières syndrome (AGS), in which the immune system attacks healthy brain cells. 12/
. ninds.nih.gov/health-informa…

. ninds.nih.gov/health-informa…

@BrainInflCollab These surprise findings suggested that ADAR1 was not only important in viral infection but was critical for keeping biological peace in the body in other ways. 13/
@BrainInflCollab So, it was really interesting that not having this RNA editing protein would suddenly cause such severe symptoms in the brain, even when there was no virus around. 14/
@BrainInflCollab It made the researchers really curious what the protein is doing to keep us healthy separately from its role in virus infection. 15/
@BrainInflCollab The team earlier discovered that ADAR1-mediated modifications made to our own dsRNA molecules keep PRRs (pattern recognition receptors) from constantly triggering inflammation in healthy tissues. 16/
cell.com/cell/fulltext/…

cell.com/cell/fulltext/…

@BrainInflCollab That finding satisfied some of the researchers’ curiosity about how PRRs avoid flagging healthy human RNA, but they still don't know exactly how ADAR1 works to hide human RNA from these receptors. 17/
@BrainInflCollab They wanted to find out why the symptoms of AGS were mostly in the brain, when RNA is found throughout the body. 18/
@BrainInflCollab If mutations in ADAR1 make the immune system recognize RNA in the brain and trigger inflammation, why didn't it also cause similarly high levels of inflammation in the heart, the liver, the blood and elsewhere? 19/ 

@BrainInflCollab In the current study, the researchers engineered stem cells to lack ADAR1 and then coaxed both normal stem cells and those without ADAR1 to develop into neurons and other cell types. 20/
@BrainInflCollab They discovered that neurons, compared to other cells, have far more long double-stranded RNA (dsRNA) structures like that found in viruses. 21/
@BrainInflCollab Without ADAR1, most cells had just small amounts of dsRNA for PRRs to flag as dangerous. Neurons, on the other hand, had a lot. Without ADAR1 to disguise the RNA, PRRs immediately triggered inflammation in neurons. 22/ 

@BrainInflCollab In turn, that inflammation can increase levels of PRRs, exacerbating the response in a difficult-to-stop cycle. So, a cascade of events led to high production of Interferons 23/
If the human dsRNA is not edited by an enzyme (ADAR1), then it will stimulate large amounts of type 1 interferon (IFN) production in the CNS.
This might be the cause of (or a contributing factor for) many neuroinflammatory and neurodegenerative interferonopathies 24/
This might be the cause of (or a contributing factor for) many neuroinflammatory and neurodegenerative interferonopathies 24/

Once you get this initial spike in inflammation, whether it's due to a virus or to an autoimmune reaction, you can get locked into this loop where pattern recognition receptors keep finding RNA and then you make more PRRs and more IFNs. 25/
This likely explains what occurs in the brains of people with AGS, but also hints at a possible mechanism for the hard-to-stop inflammation seen in conditions like lupus brain fog, ALS, and Alzheimer's disease. 26/
To try combating inflammation in their isolated neurons, the team decreased levels of free-floating dsRNA in cells.
While this did decrease inflammation, it also led to another surprise: the neurons suddenly became more susceptible to infections with Zika & HSV viruses. 27/
While this did decrease inflammation, it also led to another surprise: the neurons suddenly became more susceptible to infections with Zika & HSV viruses. 27/
The researchers realized that it's actually important for your neurons to have this low-level inflammation. 28/
This minor inflammation triggered by RNA in the brain takes on the role of a pilot light on a stove—it keeps the immune system activated and ready to react more quickly when there is an invading virus. 29/
In textbooks, we've learned that PRRs are sitting around waiting for pathogens. But this research is suggesting that maybe these molecules are always sensing some of our own RNA and keeping inflammation turned on at a very low level. 30/
nature.com/articles/s4139…

nature.com/articles/s4139…

Science is addictive. Rather than be discouraged by all the surprises in their study, they are more motivated than ever to understand the complex interplay between RNA and immune responses in the brain—and elsewhere. 31/ 

The neurodegenerative diseases are the last frontier of biomedicine where development of therapeutics has been confounding and slow.
For many of these diseases, if we can harness the immune system in the right way, we might see a breakthrough. 32/32
For many of these diseases, if we can harness the immune system in the right way, we might see a breakthrough. 32/32

• • •
Missing some Tweet in this thread? You can try to
force a refresh