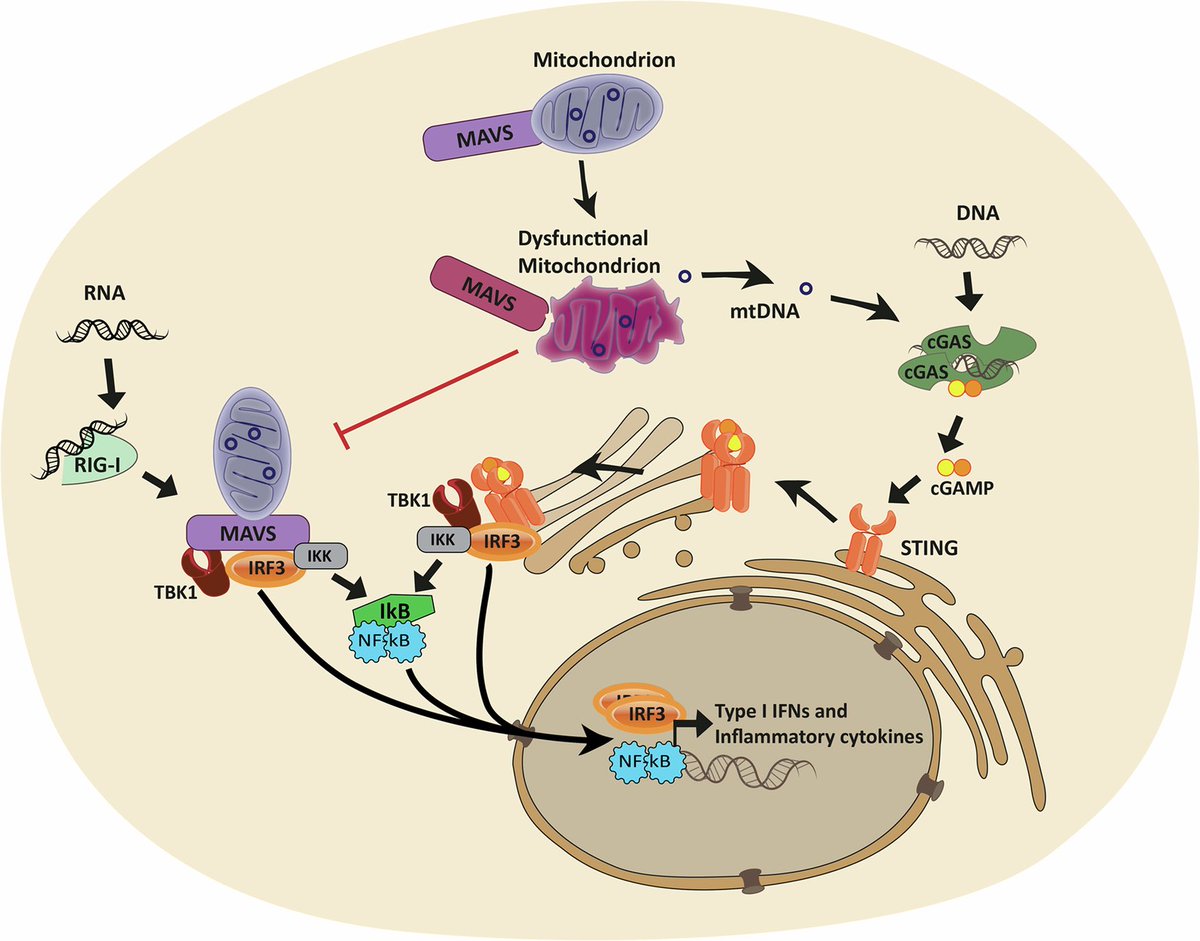
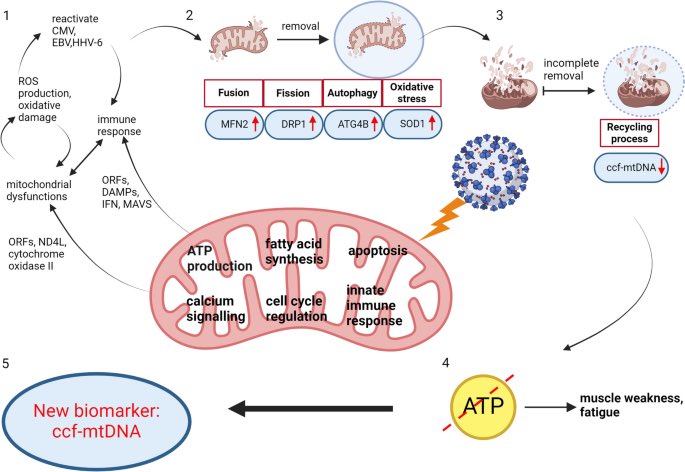
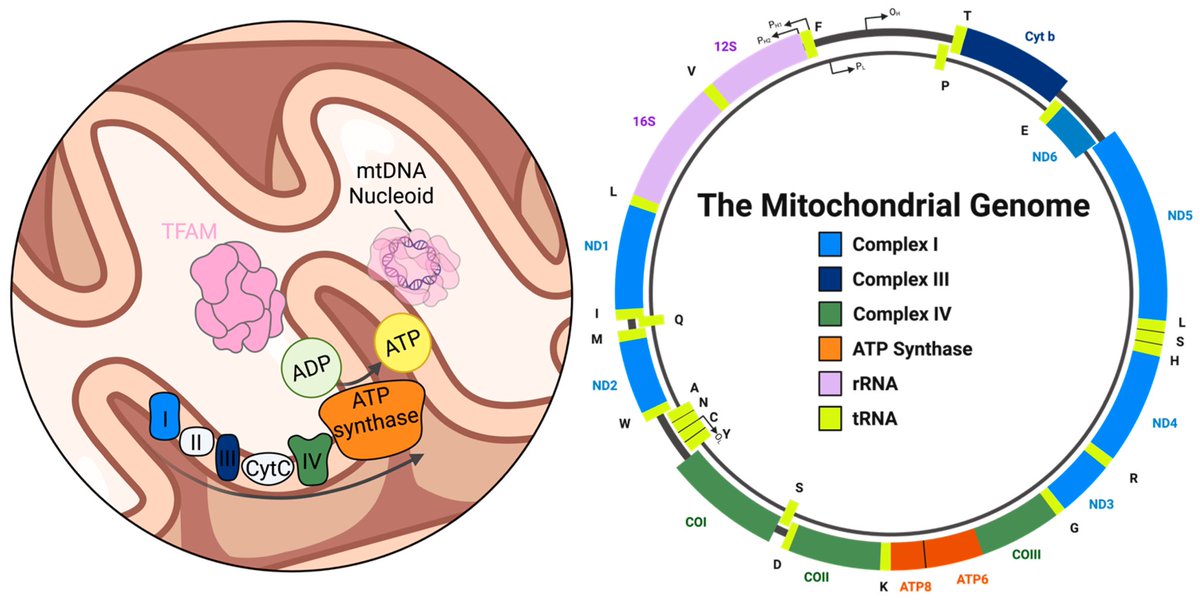
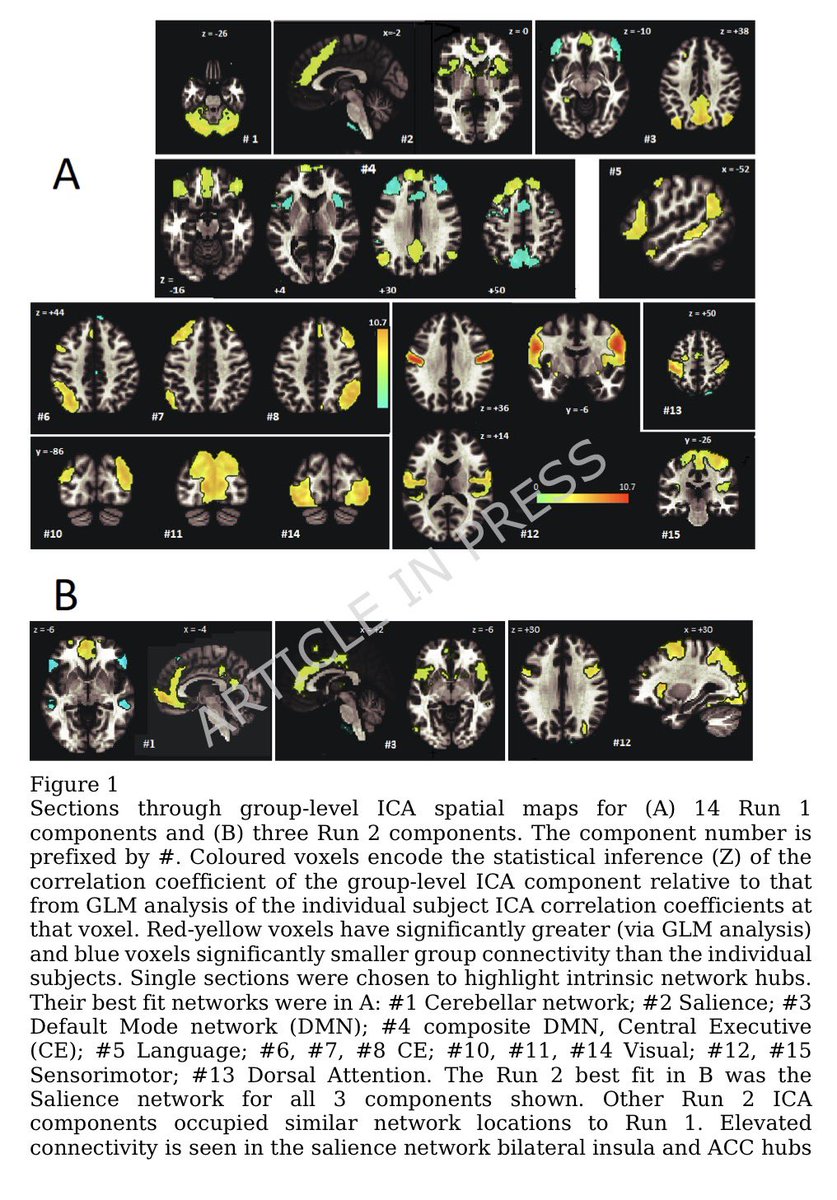
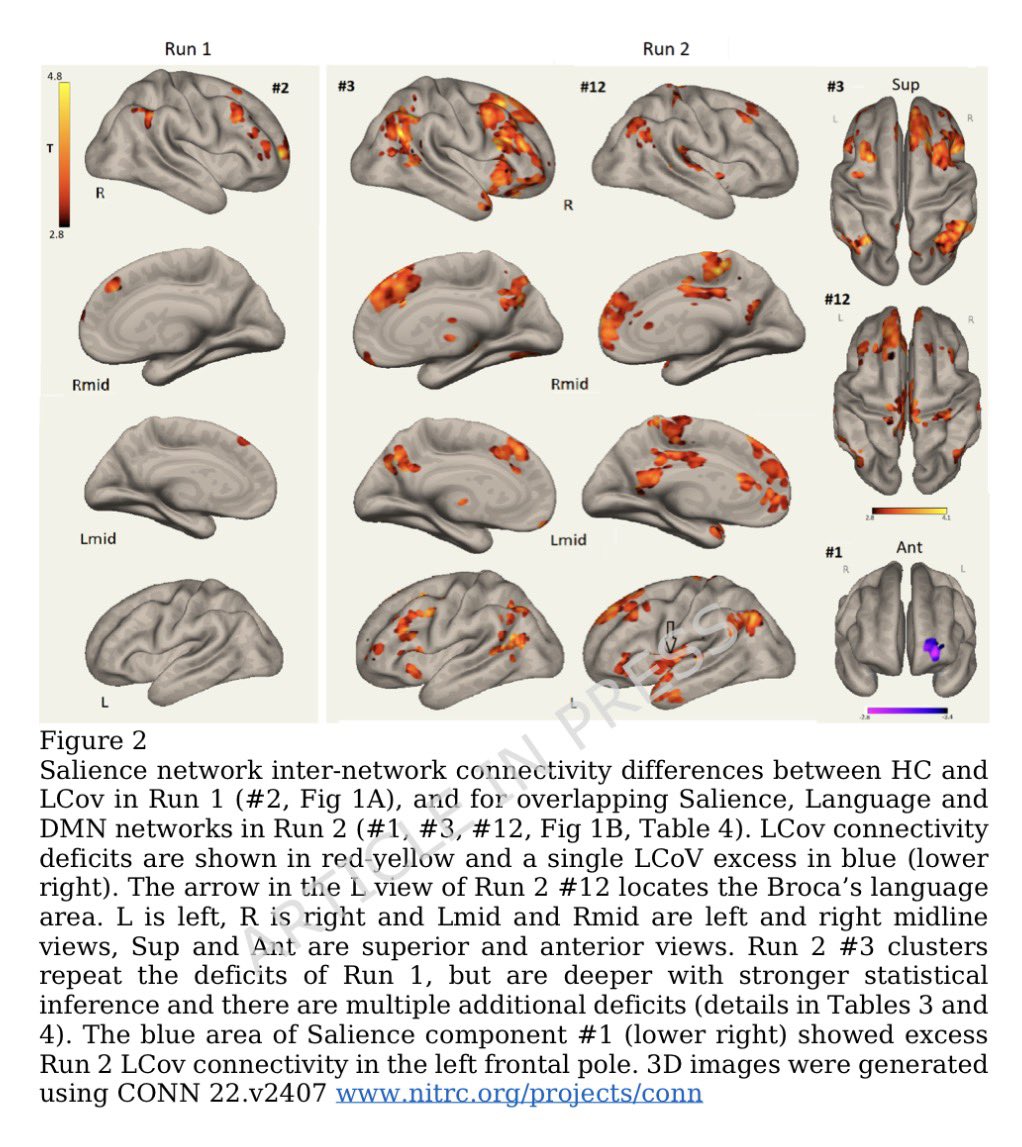
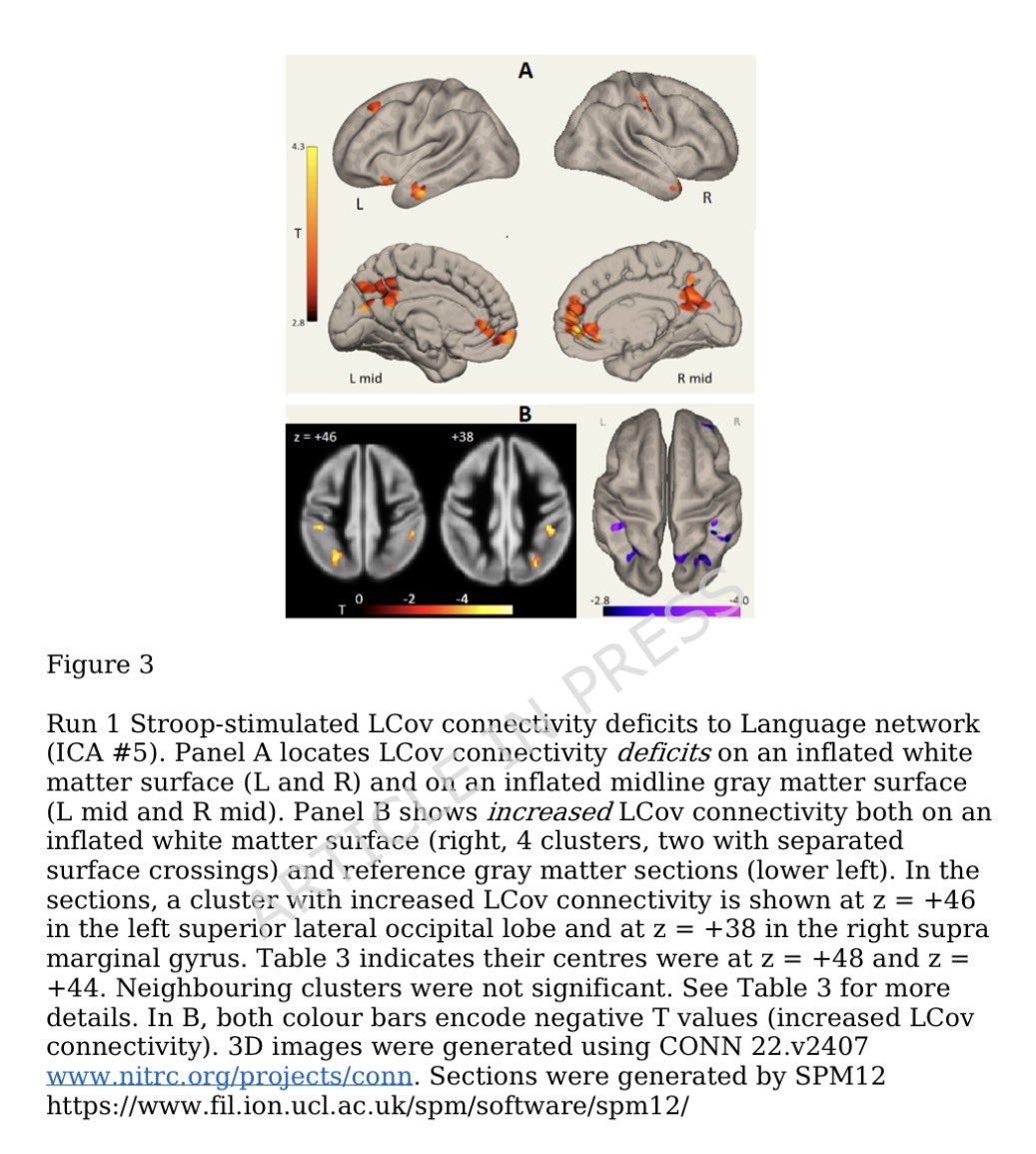
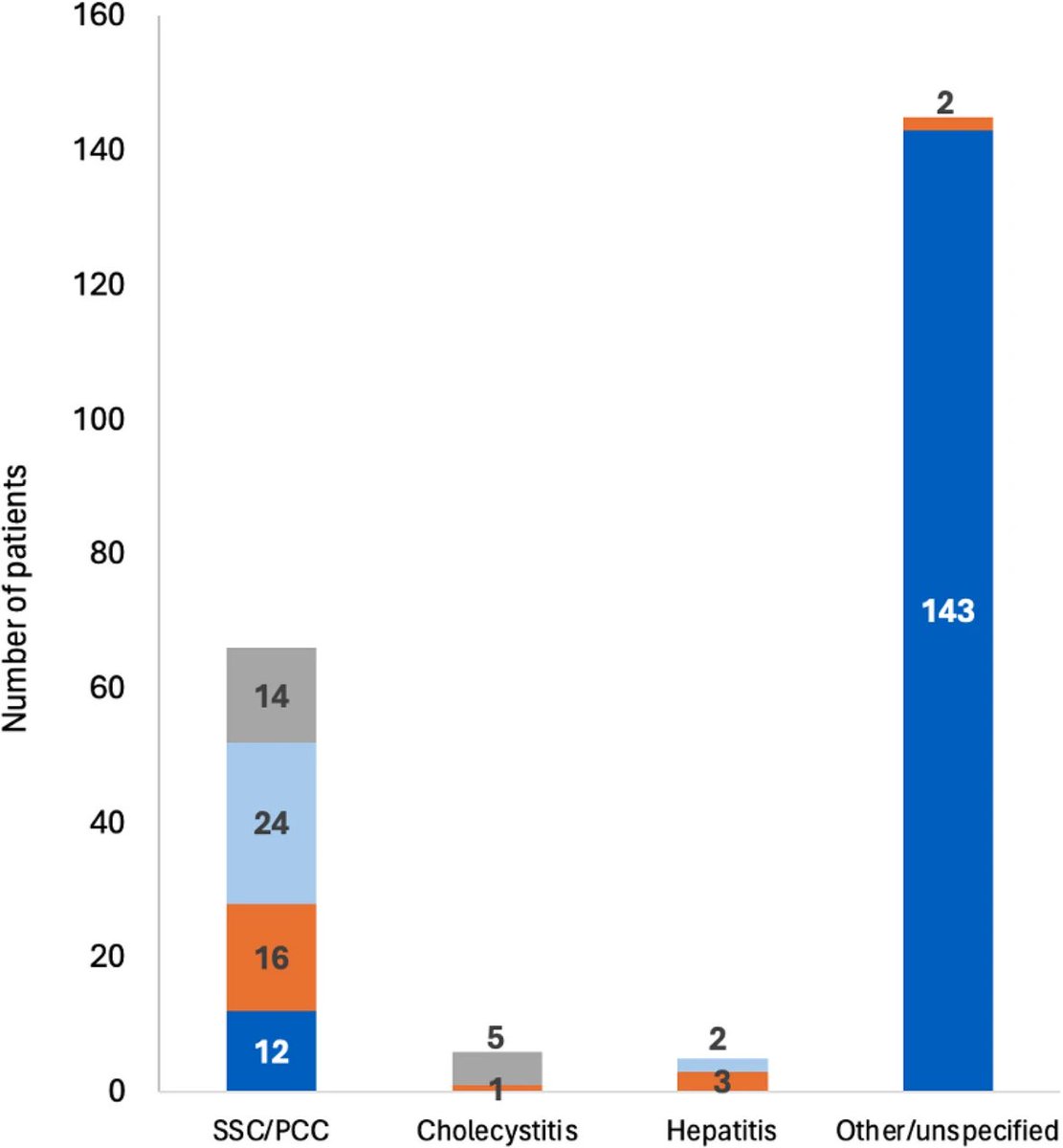
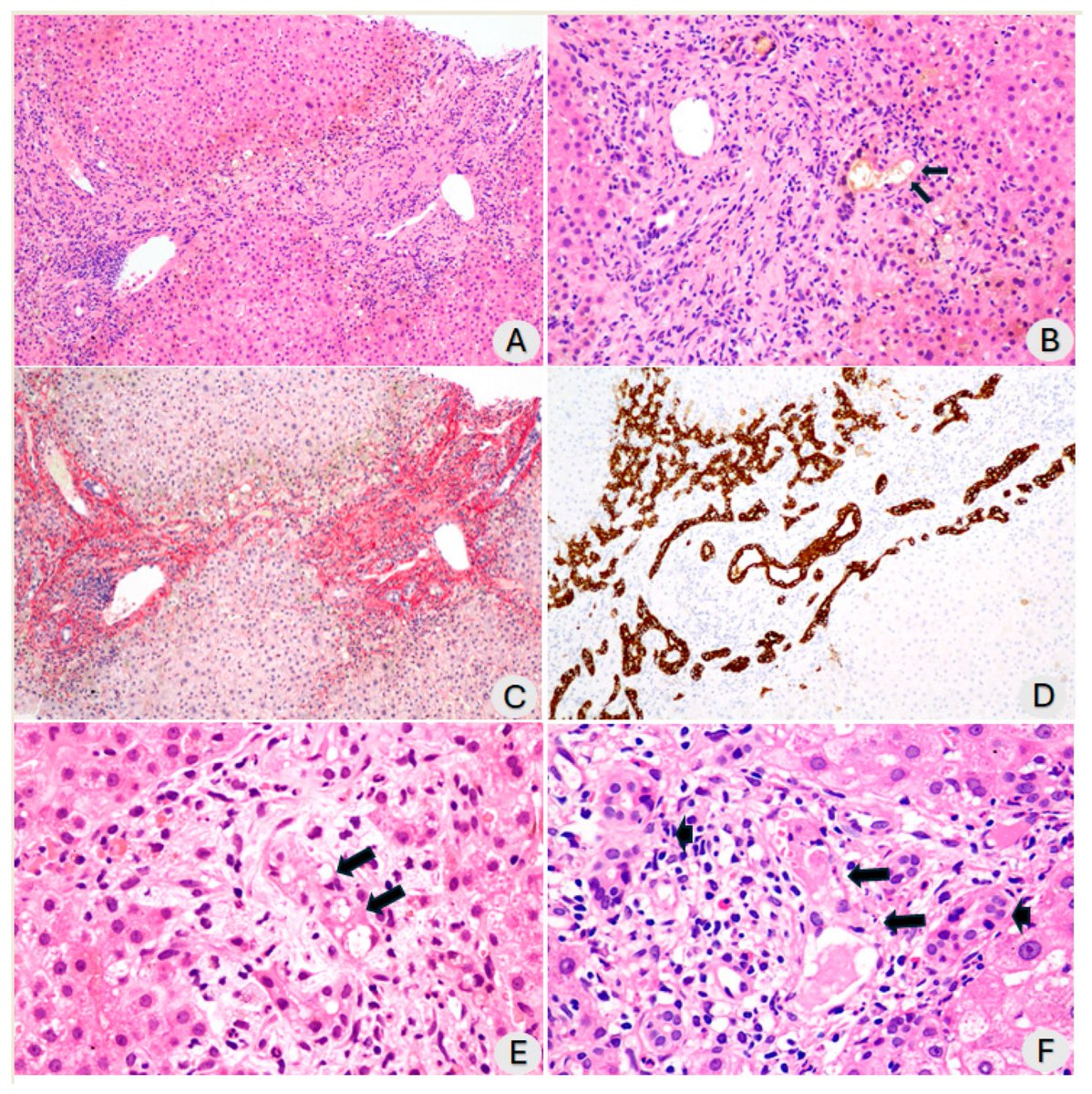
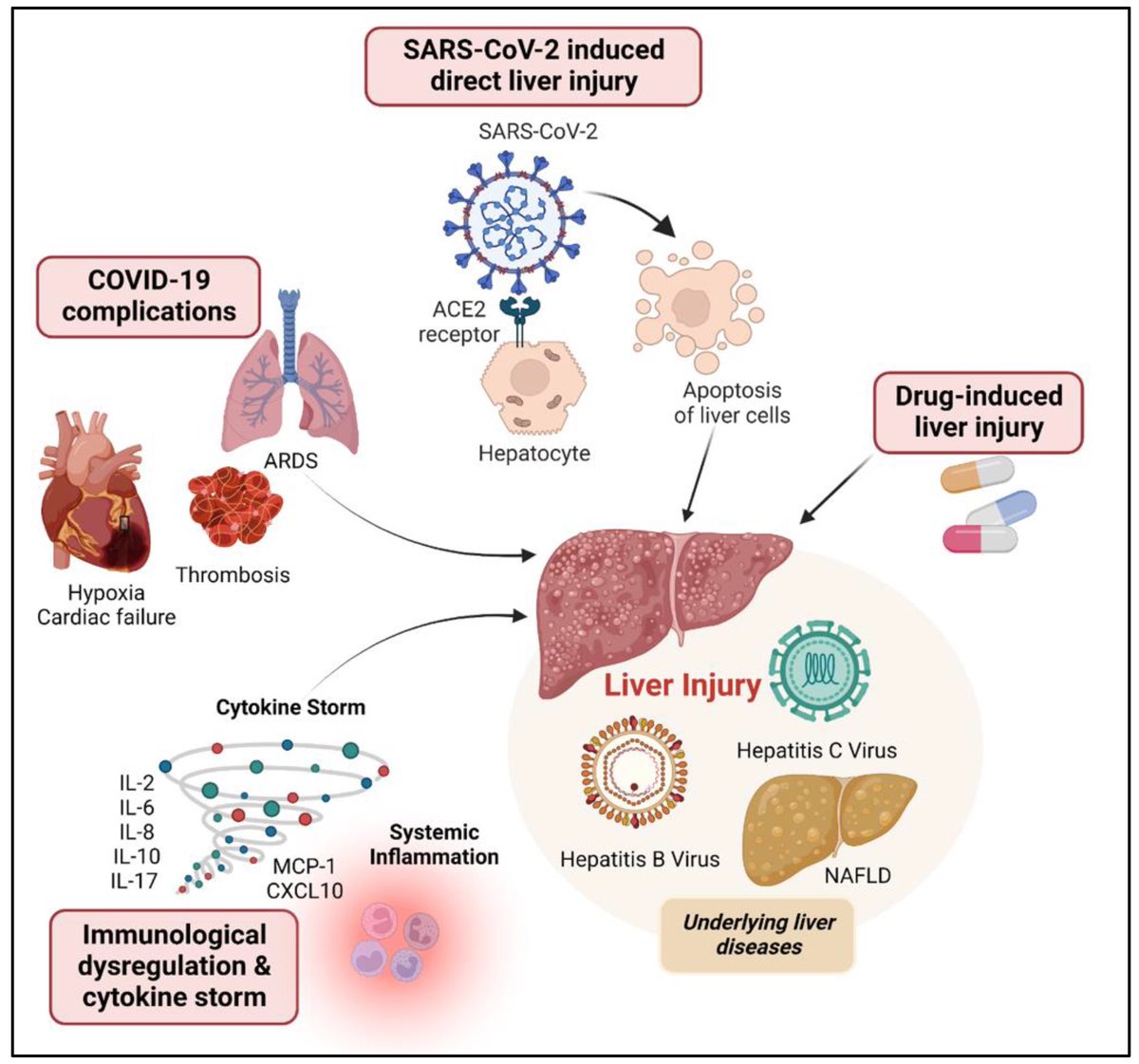
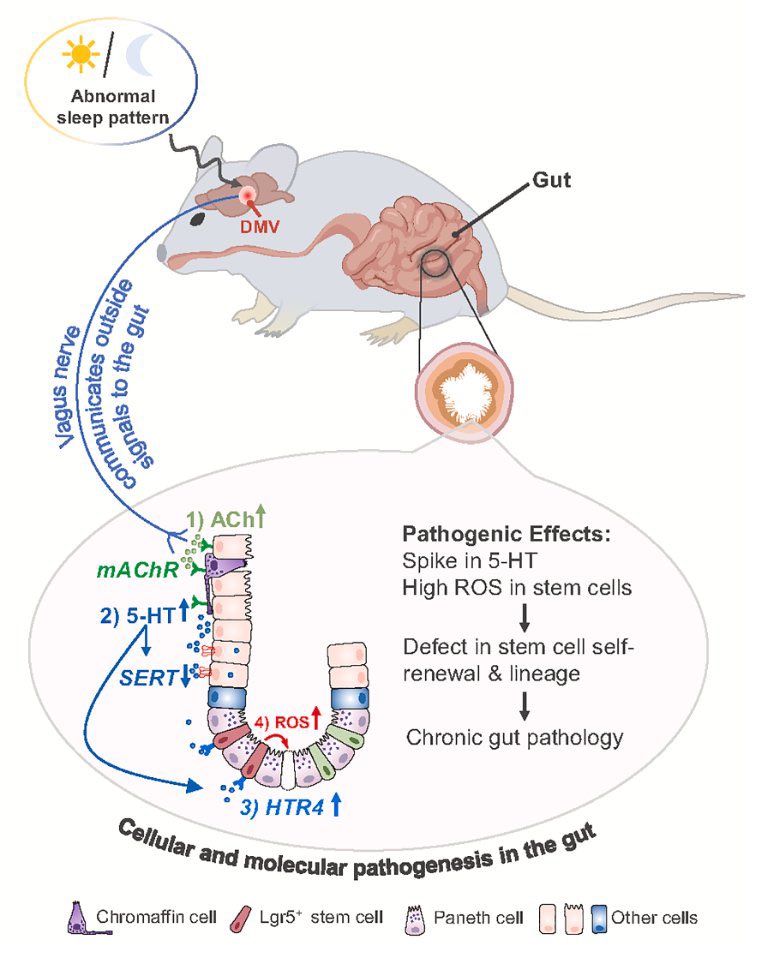
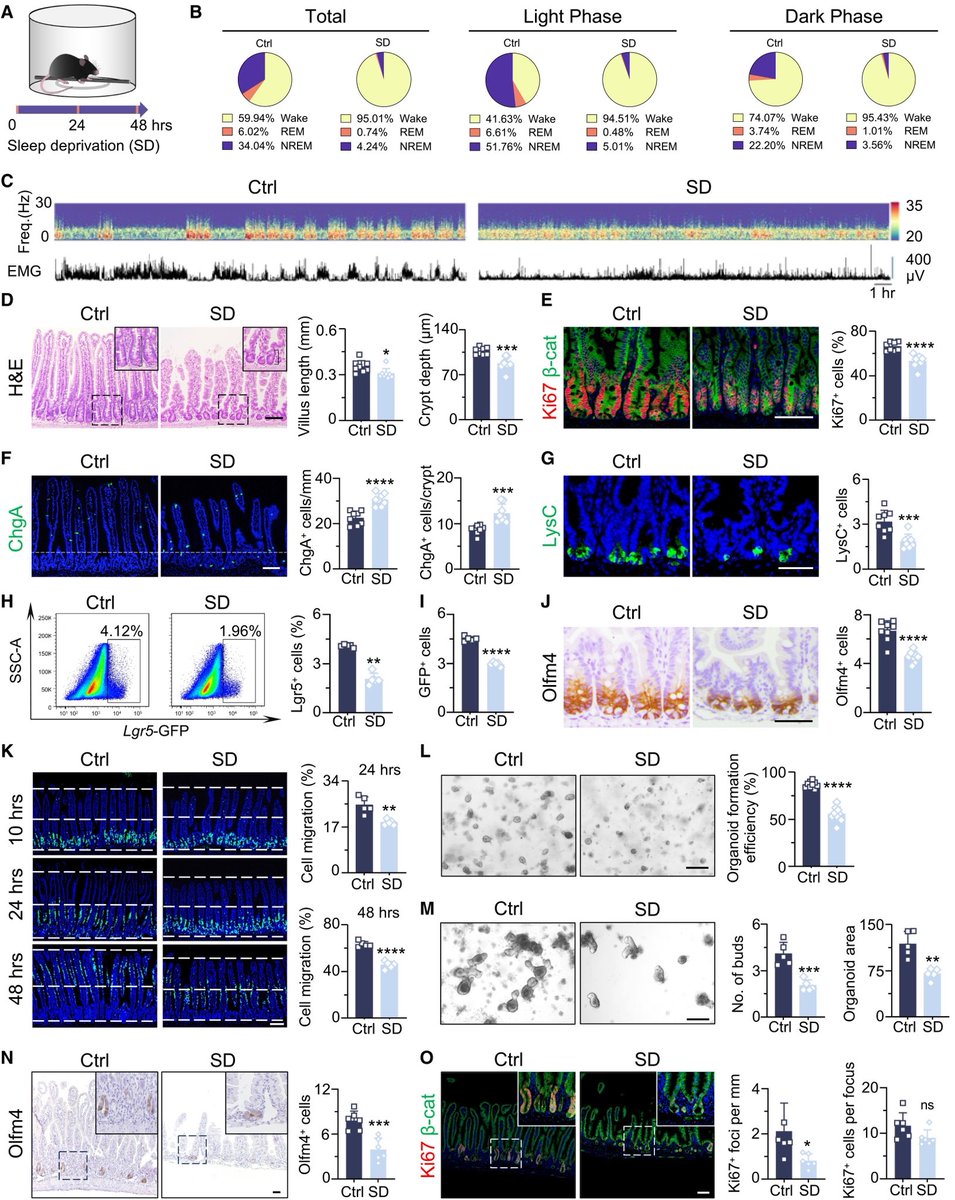
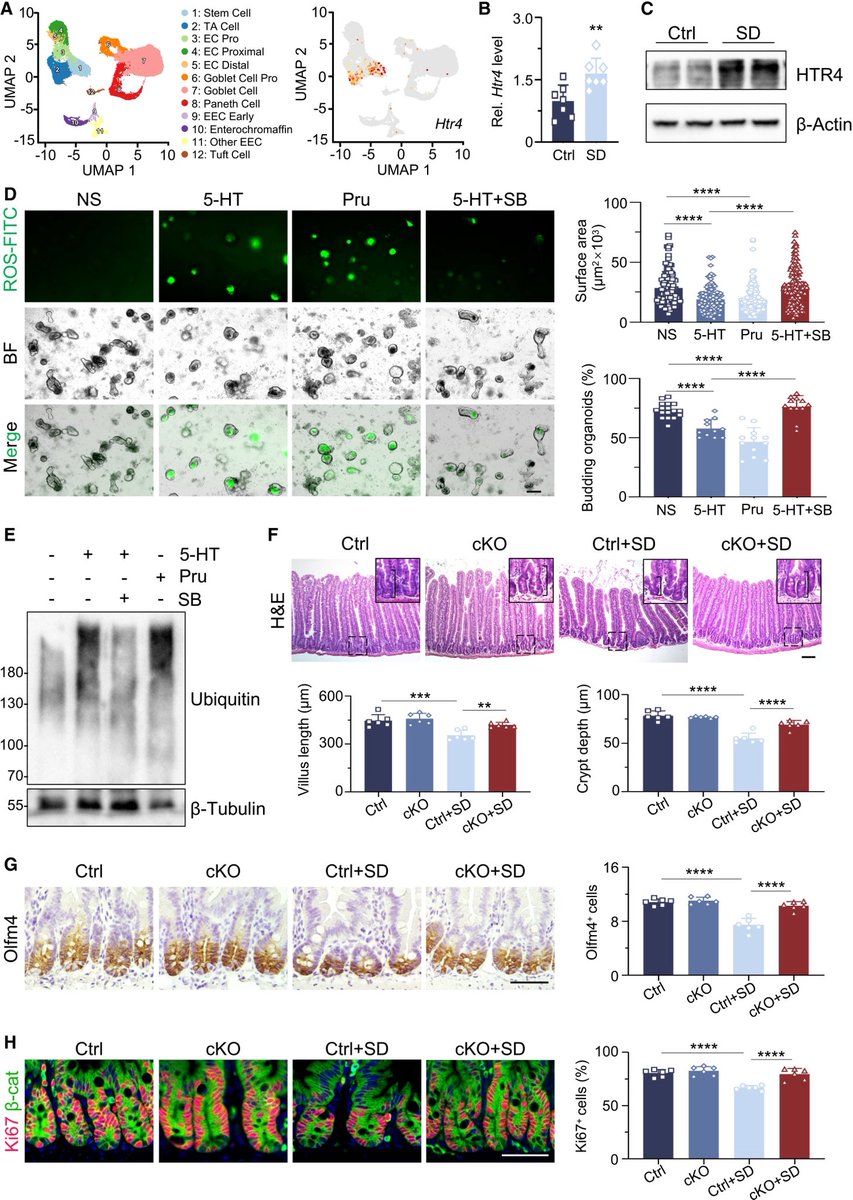
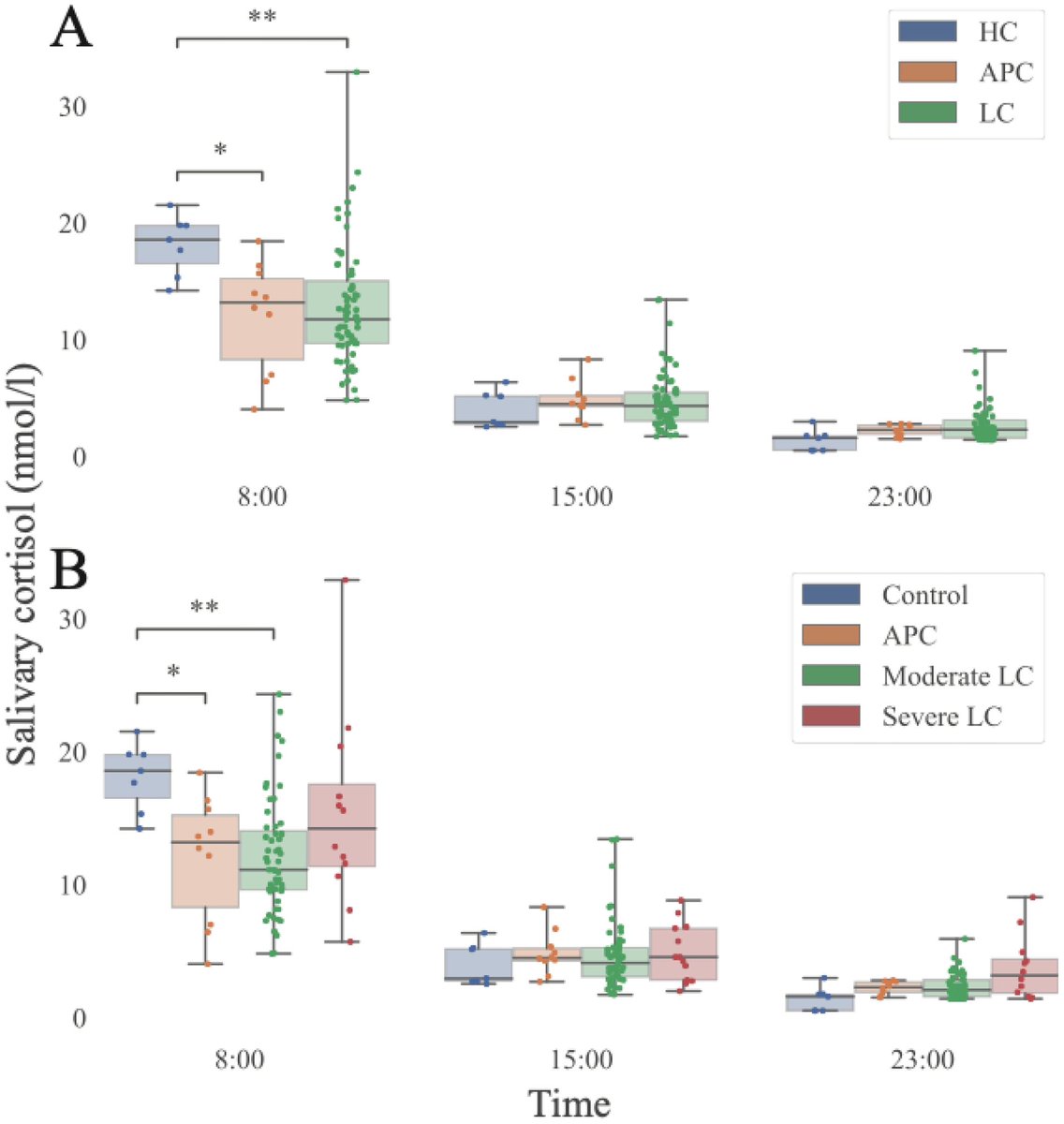
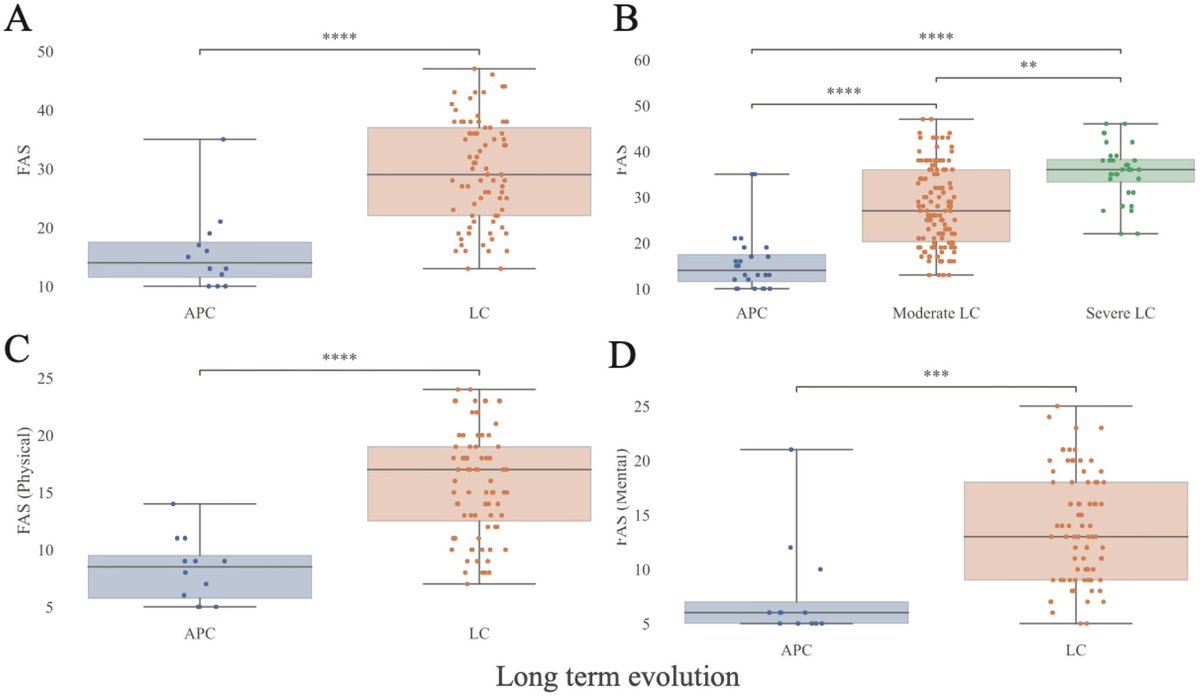
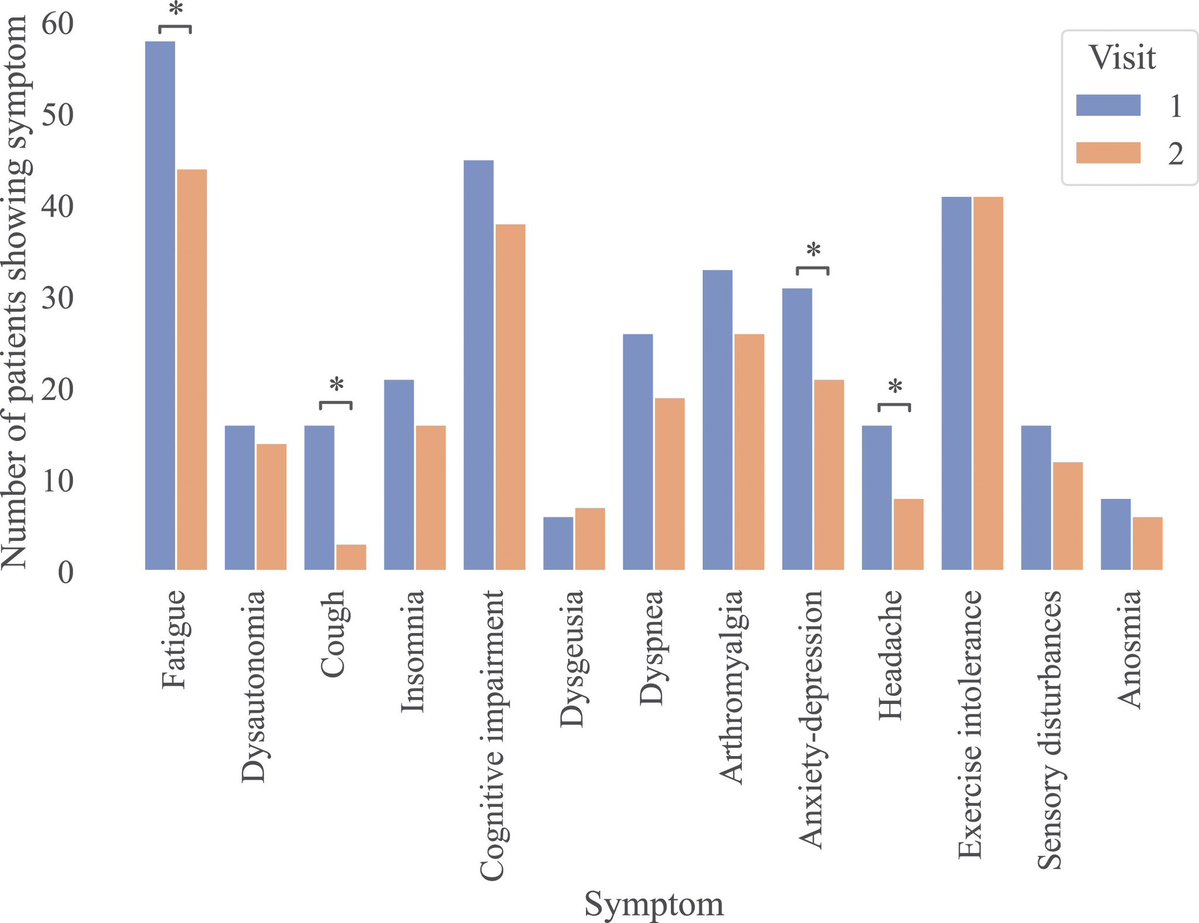
The molecular origins of neuro-COVID, and how it contributes to #LongCovid (PASC) are unknown, however a growing body of research highlights that the self-assembly of protein fragments from SARS-CoV-2 into amyloid nanofibrils may play a causative role. 1/ 

Earlier, researchers identified two fragments from the SARS-CoV-2 proteins, ORF 6 & ORF10, that self-assemble into neurotoxic amyloid assemblies.
Here they show the self-assembly mechanisms & nano-architectures formed by these fragments and their biological responses. 2/
Here they show the self-assembly mechanisms & nano-architectures formed by these fragments and their biological responses. 2/

By solubilising the peptides in a fluorinated solvent, they eliminated insoluble aggregates in the starting materials (seeds) that change the polymorphic landscape of the assemblies. 3/
The resultant assemblies are dominated by structures with higher free energies (e.g. ribbons and amorphous aggregates) that are less toxic to cultured neurons but do affect their mitochondrial respiration. 4/ 

This work highlights the importance of understanding the polymorphic behaviour of amyloids and the correlation to neurotoxicity, particularly in the context of neuro-COVID & PASC. 6/6
pubs.rsc.org/en/Content/Art…


pubs.rsc.org/en/Content/Art…


• • •
Missing some Tweet in this thread? You can try to
force a refresh