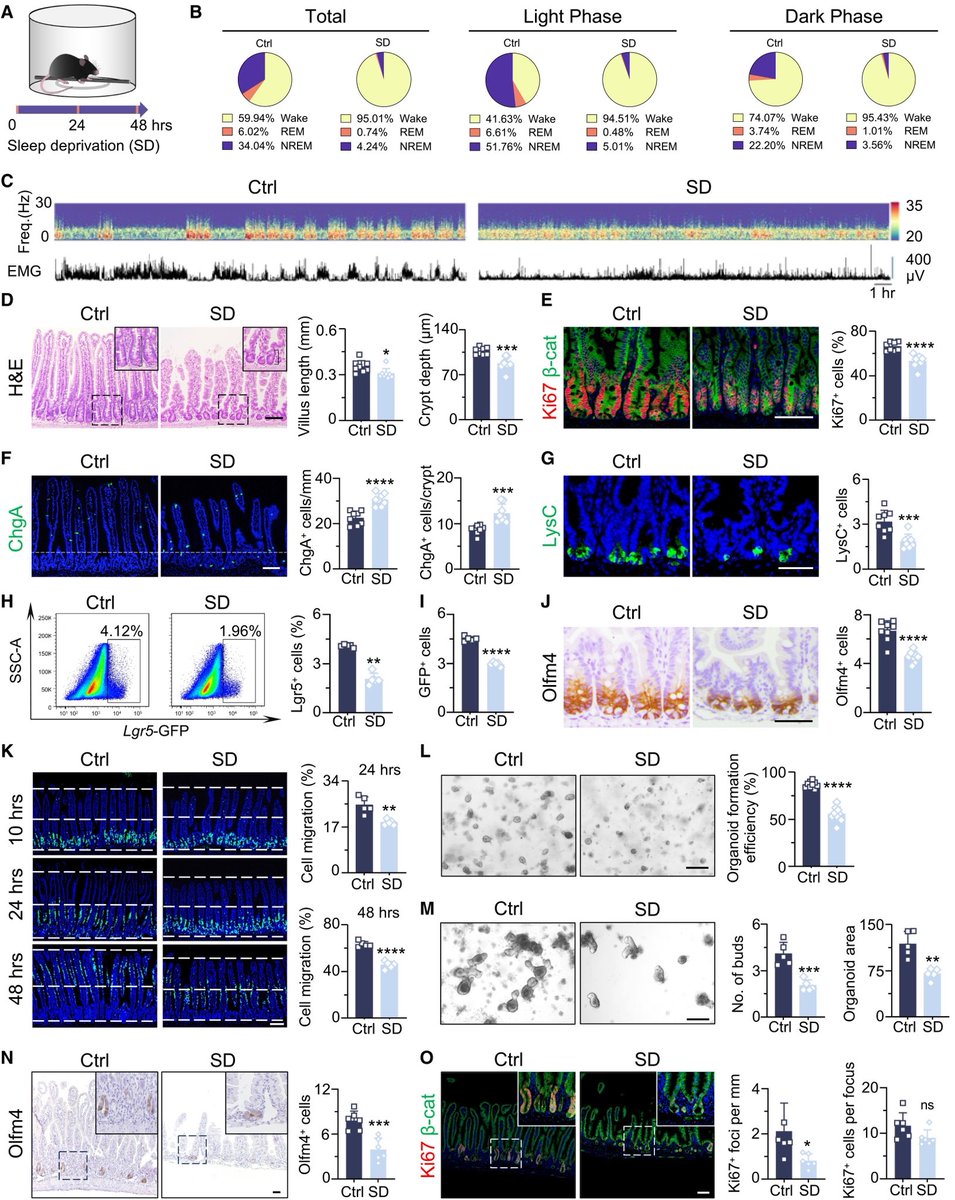
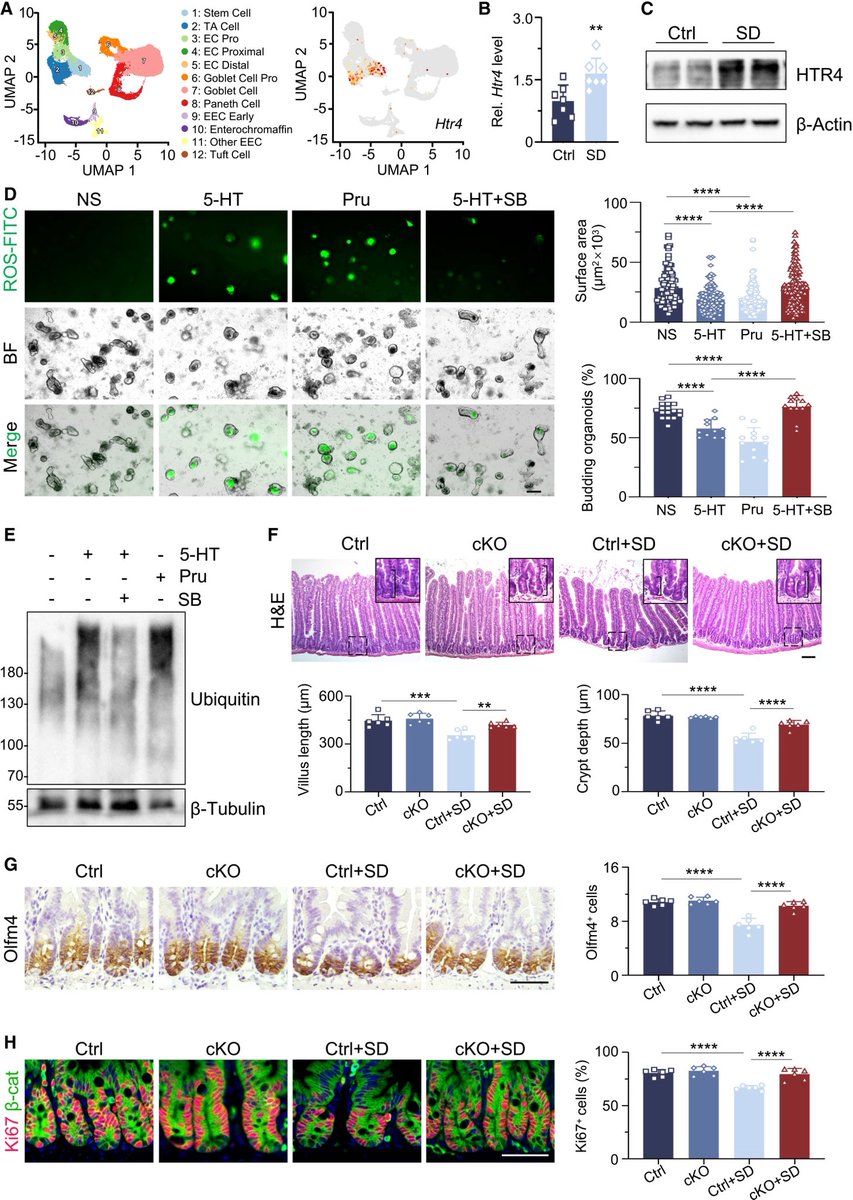
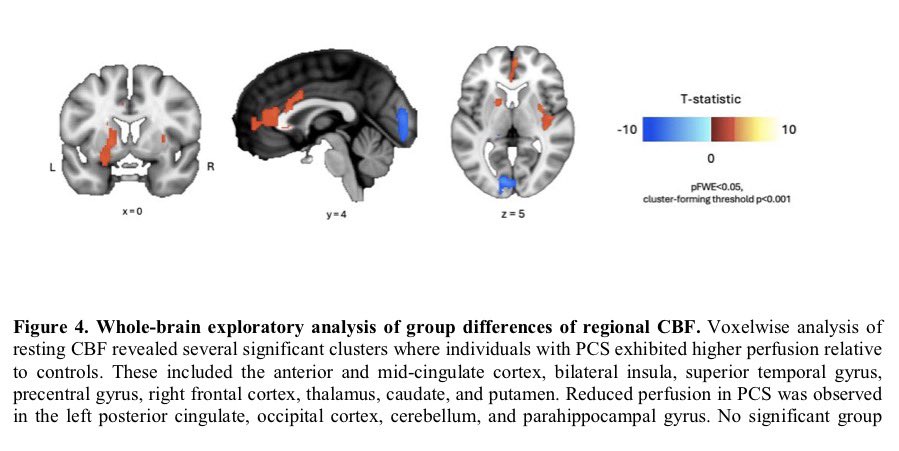
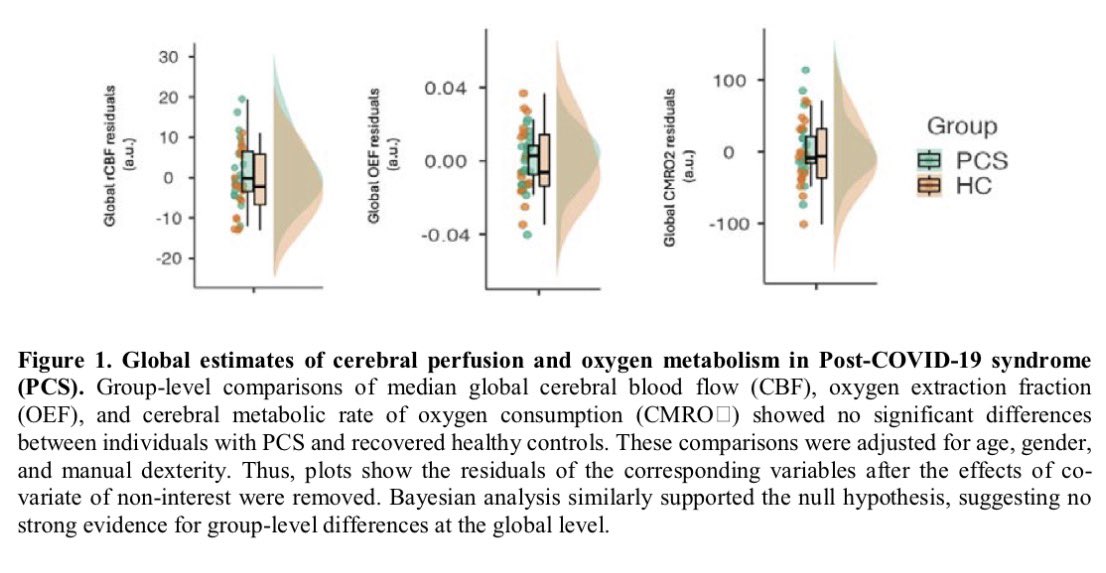
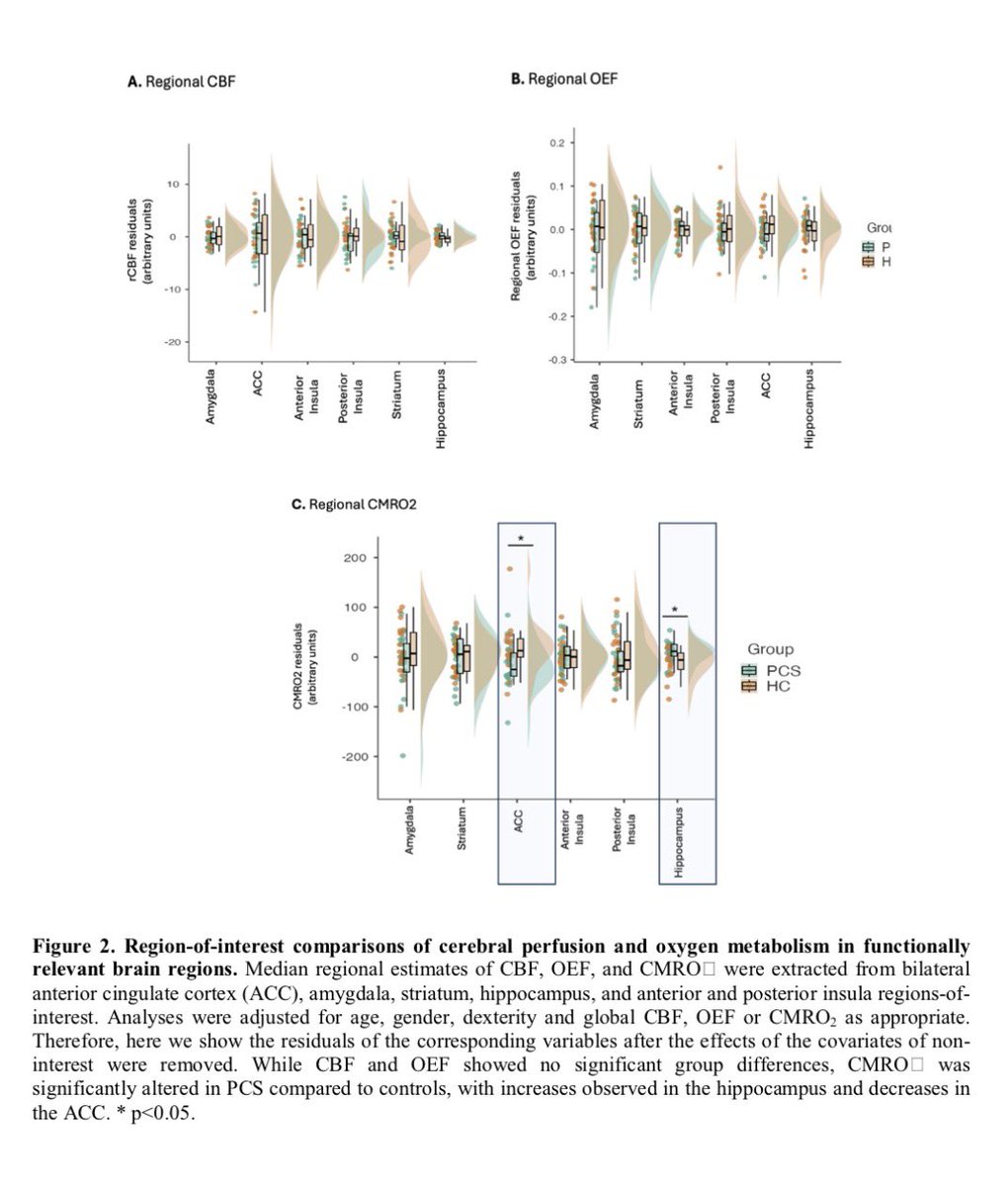
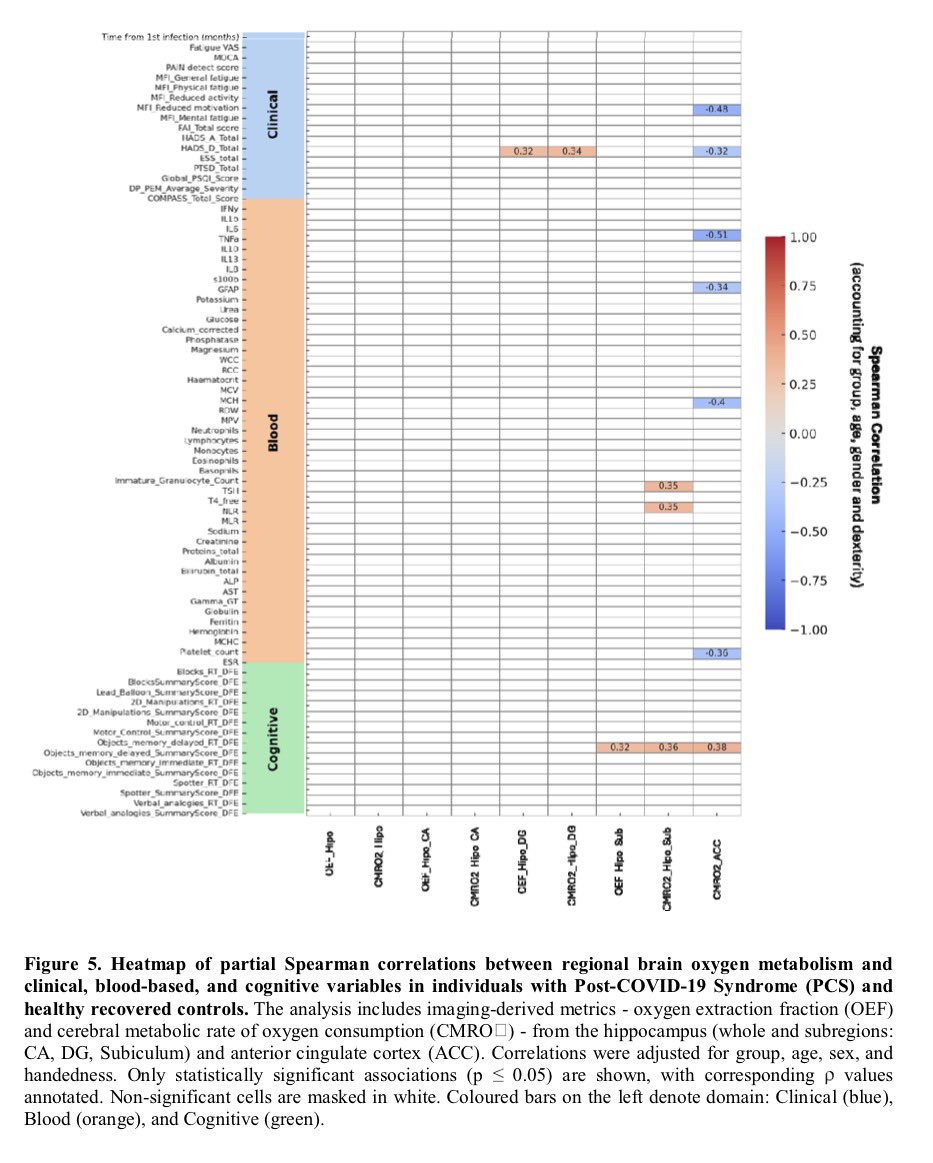
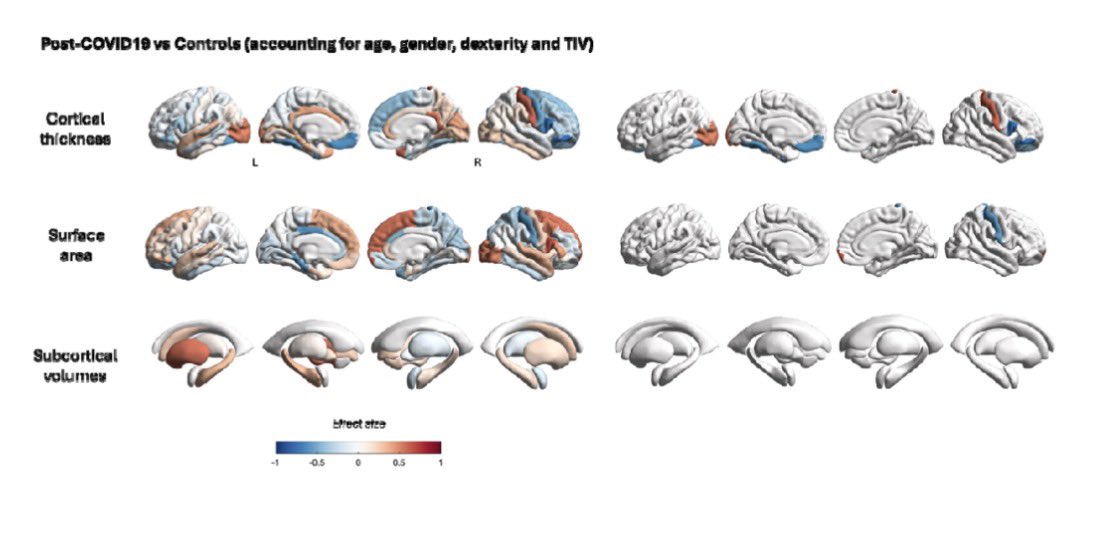
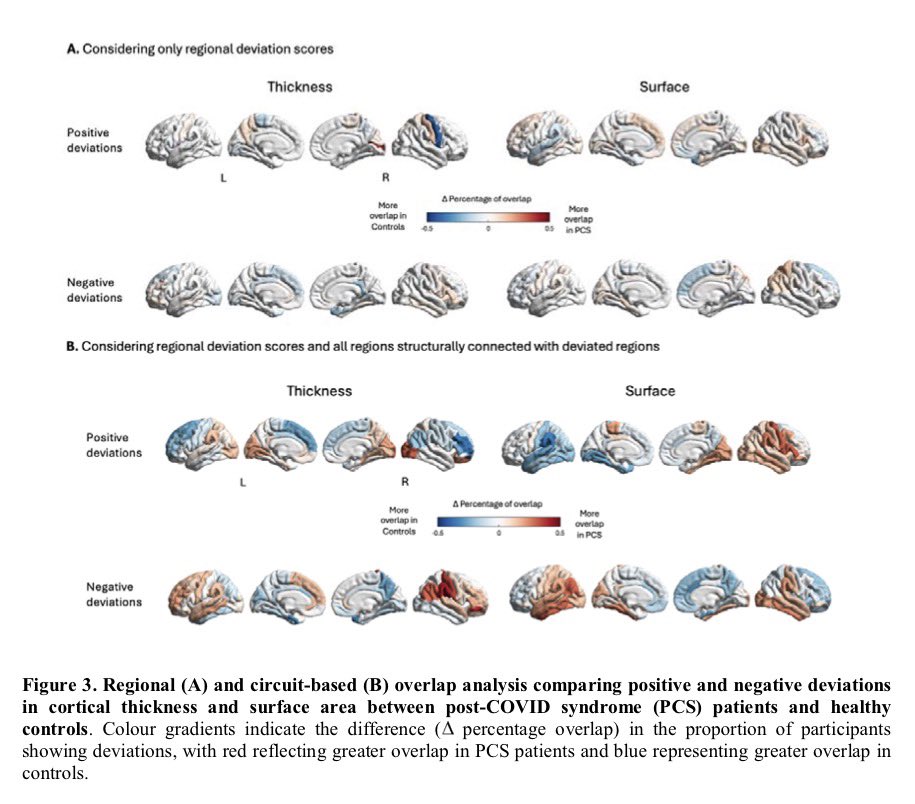
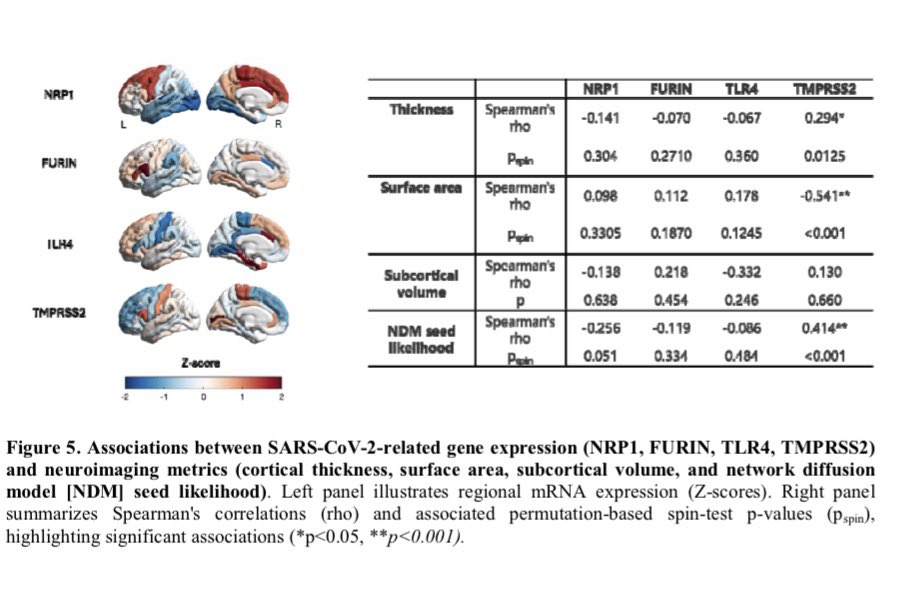
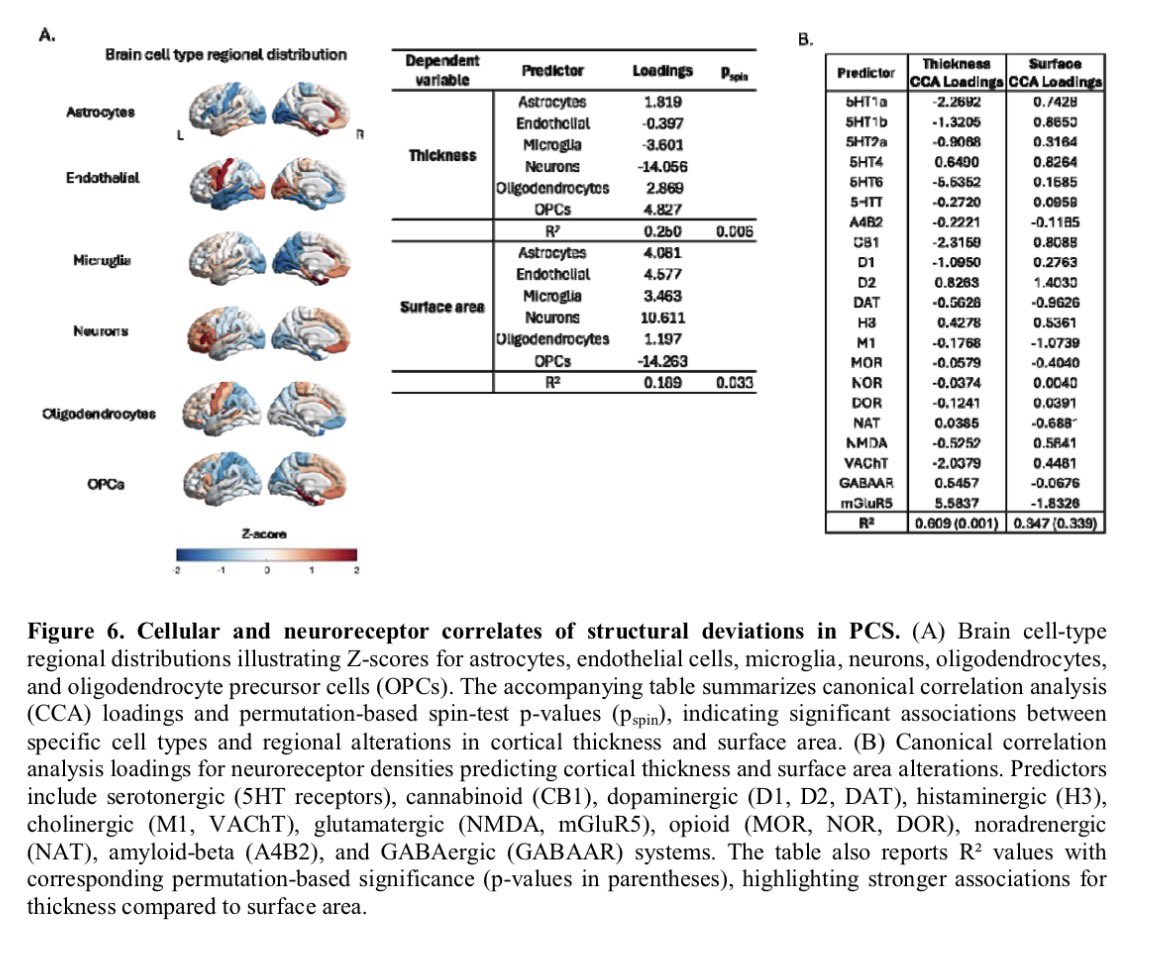
Repeated COVID vaccinations enhance mucosal immunity against the virus!
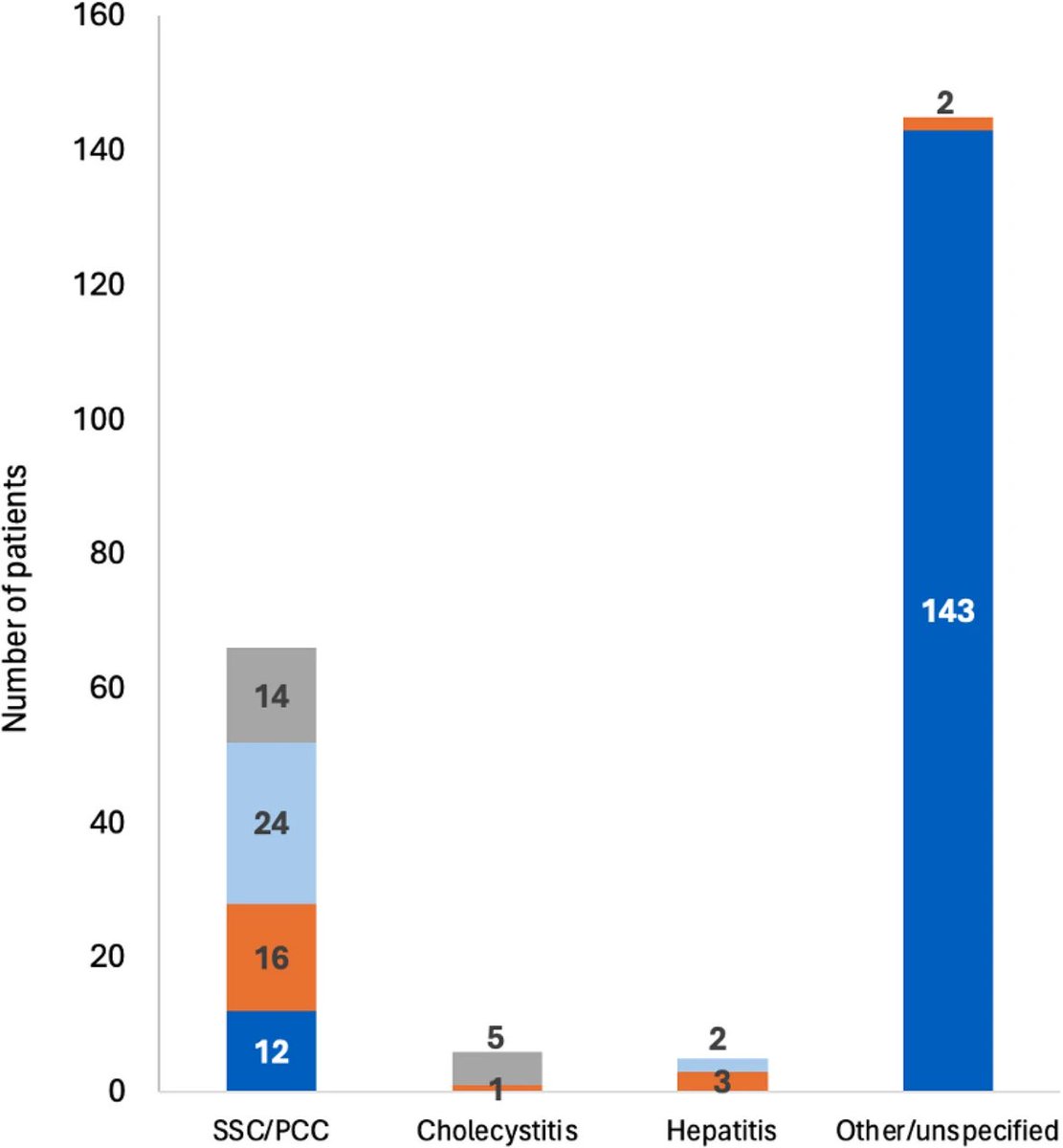
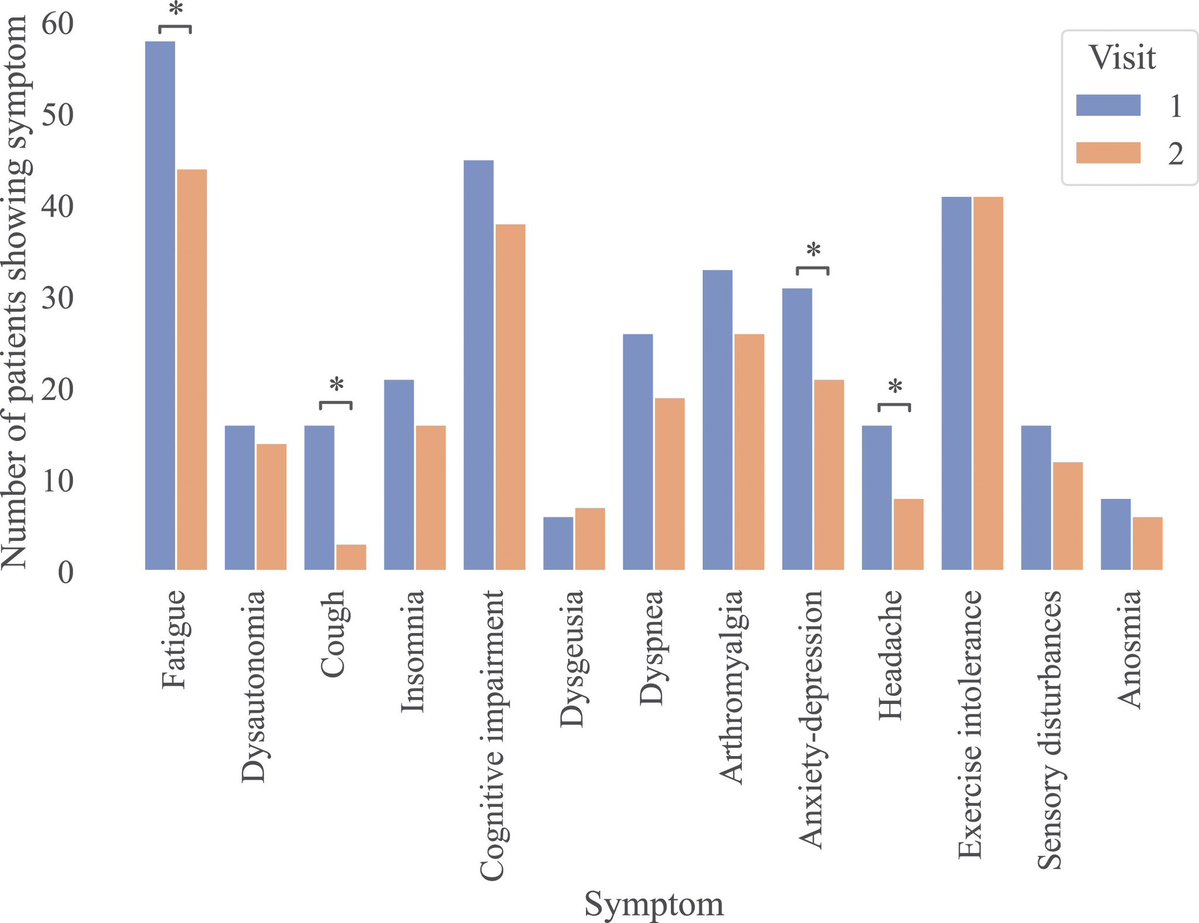
A NEW study finds that individuals who received multiple doses of mRNA vaccines exhibited a marked increase in neutralizing antibodies in nasal secretions, which are essential for blocking viral entry. 1/
A NEW study finds that individuals who received multiple doses of mRNA vaccines exhibited a marked increase in neutralizing antibodies in nasal secretions, which are essential for blocking viral entry. 1/

Not only that, but the immune responses generated by mRNA vaccines may persist longer than previously thought, which provides hope for sustained protection against emerging variants of the virus. 2/ 

They found that most mucosal neutralizing antibodies were of systemic origin, w/ antibodies circulating in blood migrating to respiratory mucosa in the nose, suggesting that repeated vaccination stimulates systemic antibody production that can reach mucosal membranes. 3/ 

This study provides compelling evidence that repeated mRNA vaccinations can improve mucosal antibody responses, or stimulate pre-existing infection induced mucosal responses, which are vital for preventing infection at the entry points of the virus. 4/ 

These findings advance our understanding of mRNA vaccine–induced immunity and have implications for the design of vaccine strategies to combat respiratory infections. 5/
science.org/doi/10.1126/sc…
science.org/doi/10.1126/sc…
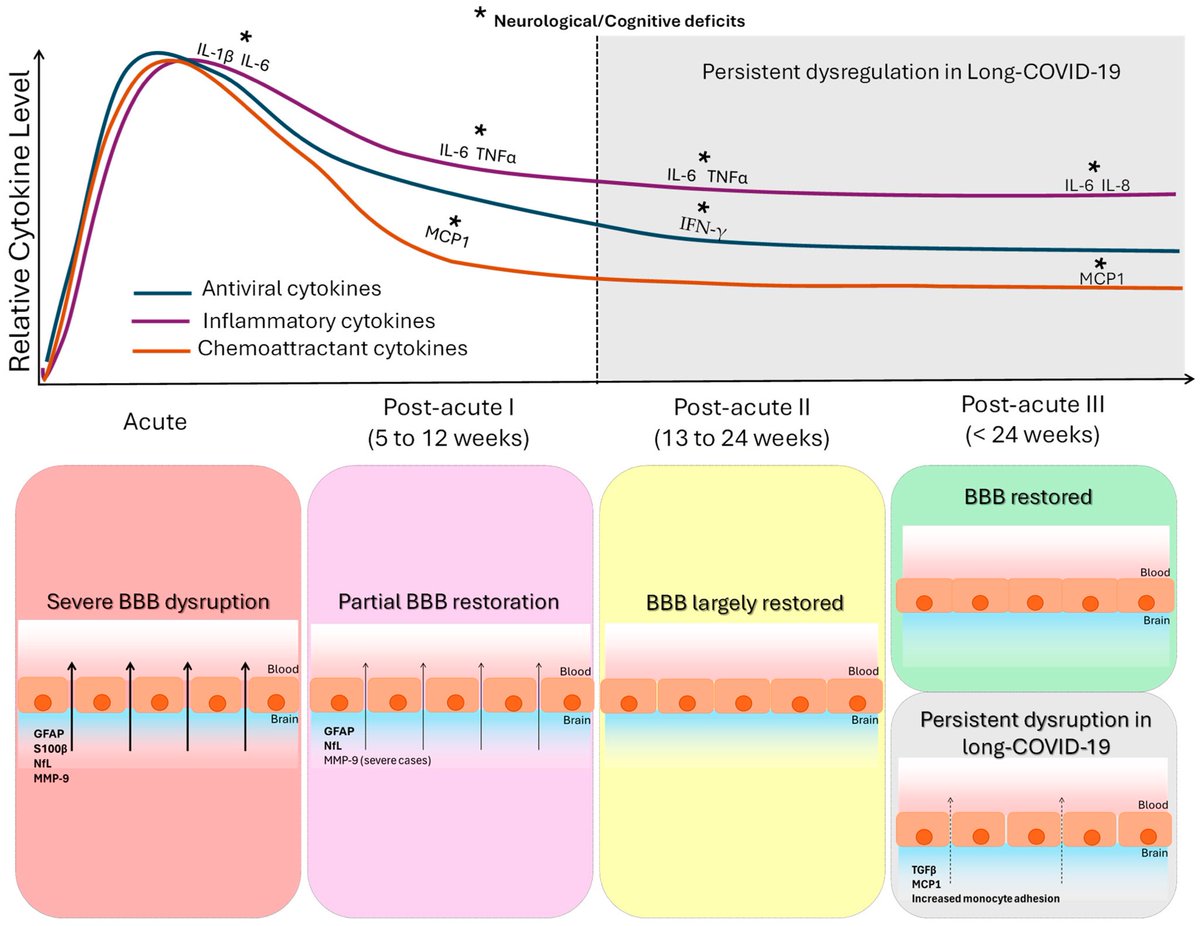
However, another new study by Lasrado et al. observed no obvious increase in neutralizing antibody titers after XBB.1.5 mRNA booster vaccination. 6/
science.org/doi/10.1126/sc…
science.org/doi/10.1126/sc…

The researchers show that XBB.1.5 mRNA boosters result in increased serum neutralization to multiple SARS-CoV-2 variants in humans, including the dominant circulating variant JN.1. 7/ 

In contrast, they found that XBB.1.5 mRNA booster did not augment mucosal NAbs or mucosal IgA responses, although acute SARS-CoV-2 XBB infection substantially increased mucosal antibody responses. 8/ 



The Lasrado et al. study shows that current XBB.1.5 mRNA boosters substantially enhance peripheral antibody responses but do not robustly increase mucosal antibody responses. 9/ 

These differing results by two studies may be due to the number of SARS-CoV-2 vaccinations or exposures, time since last exposure, and experimental approaches, but this pair of papers underscores the need to better understand the mucosal immune response in humans. 10/10
• • •
Missing some Tweet in this thread? You can try to
force a refresh