Our new study shows that SARS-CoV-2 spike protein accumulates & persists in the body for years after infection, especially in the skull-meninges-brain axis, potentially driving long COVID. mRNA vaccines help but cannot stop it🔬🧠🦠🧵👇@cellhostmicrobe cell.com/cell-host-micr…
2/n Summary: (Your weekend read:))
We found SARS-CoV-2 spike protein in the skull-meninges-brain axis in mouse models and human post-mortem tissues long after their COVID, which was associated with vascular, inflammatory changes in the brain along with neuronal damage.
We found SARS-CoV-2 spike protein in the skull-meninges-brain axis in mouse models and human post-mortem tissues long after their COVID, which was associated with vascular, inflammatory changes in the brain along with neuronal damage.

3/n Approach:
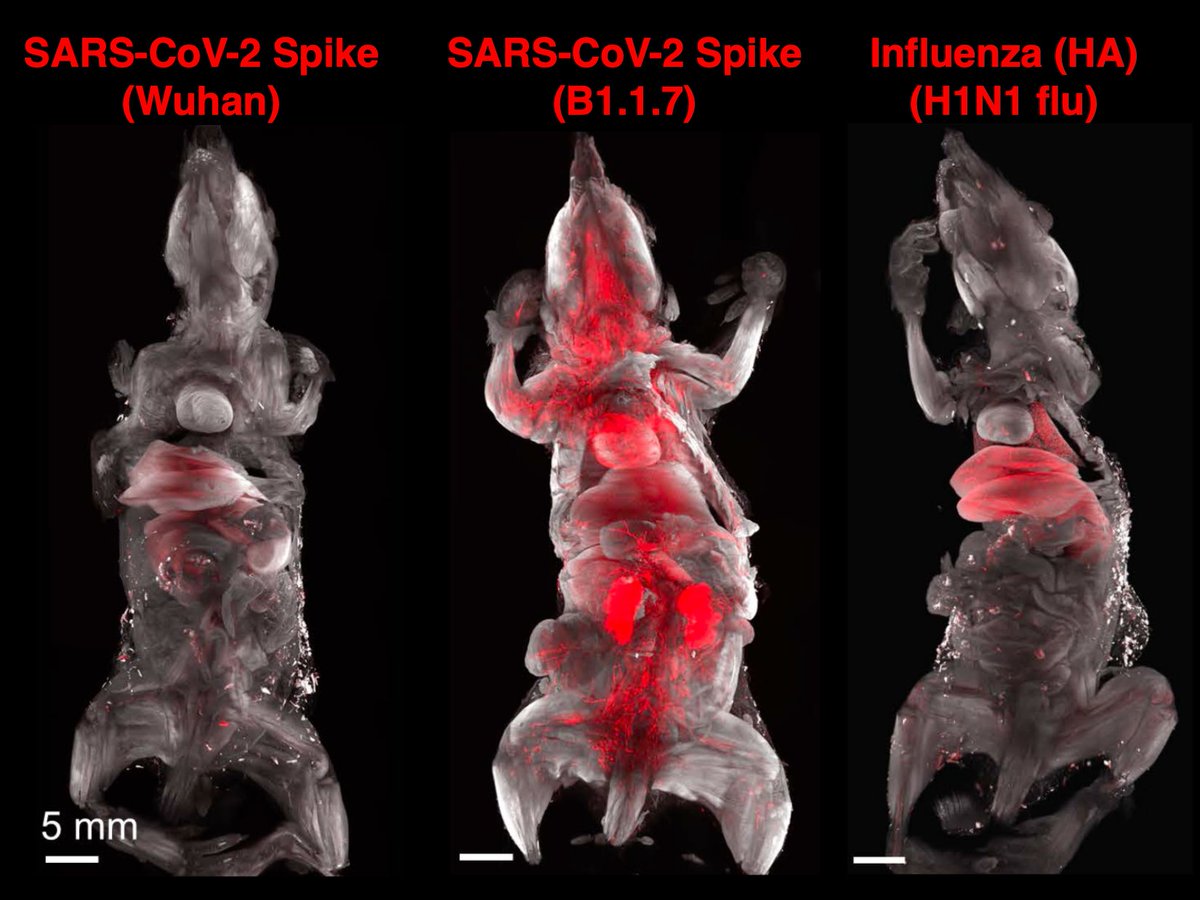
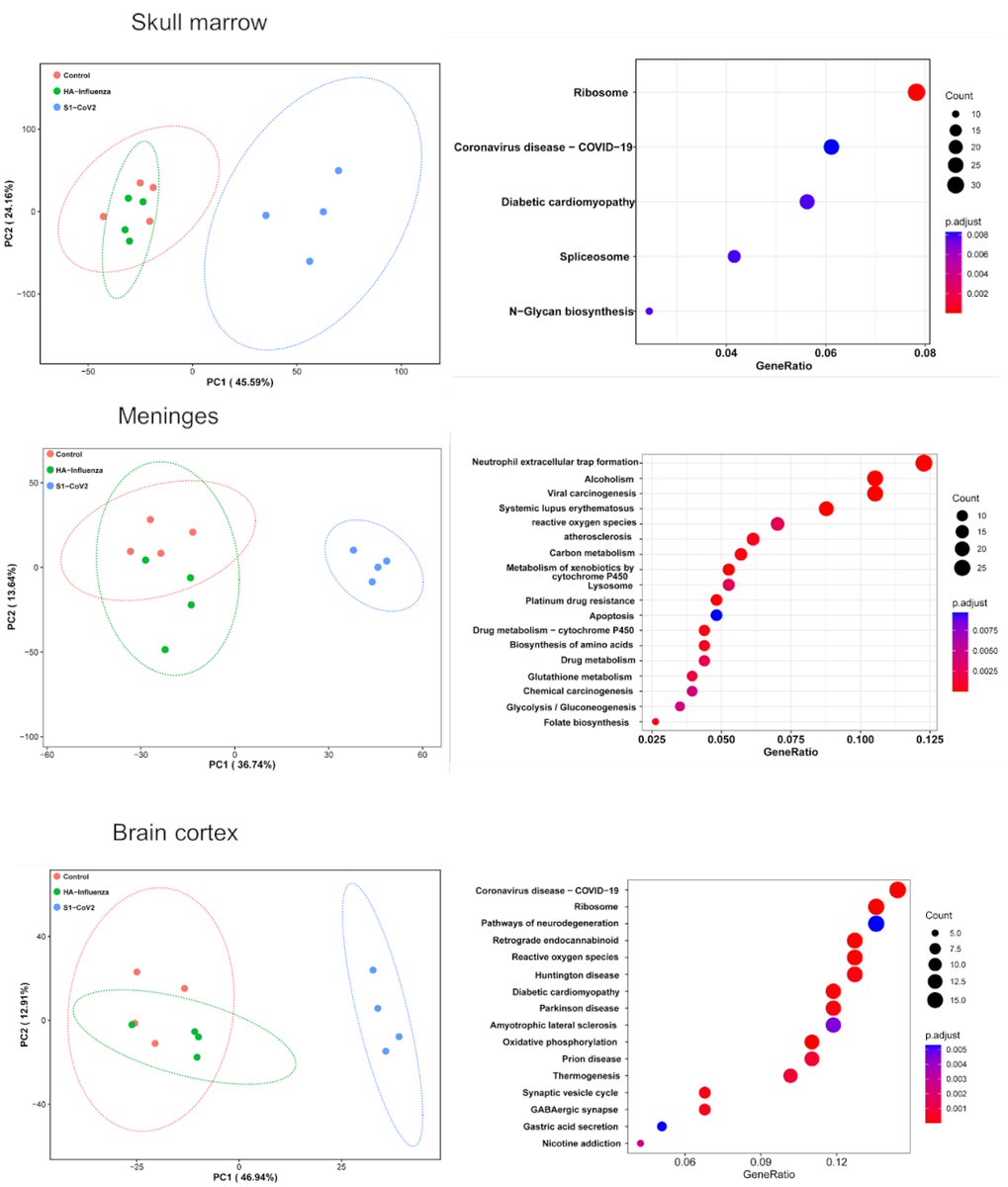
To discover all tissues that are targeted by SARS-CoV-2, we used unbiased DISCO clearing technology and mapped tissues hit by coronavirus spike vs. Influenza HA proteins (flu).
To discover all tissues that are targeted by SARS-CoV-2, we used unbiased DISCO clearing technology and mapped tissues hit by coronavirus spike vs. Influenza HA proteins (flu).
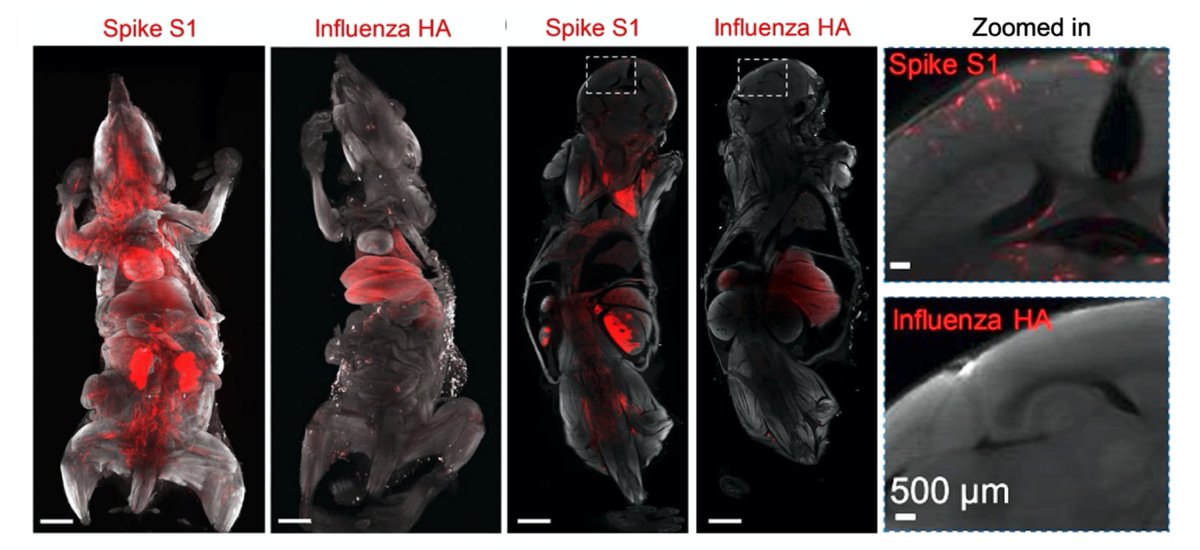
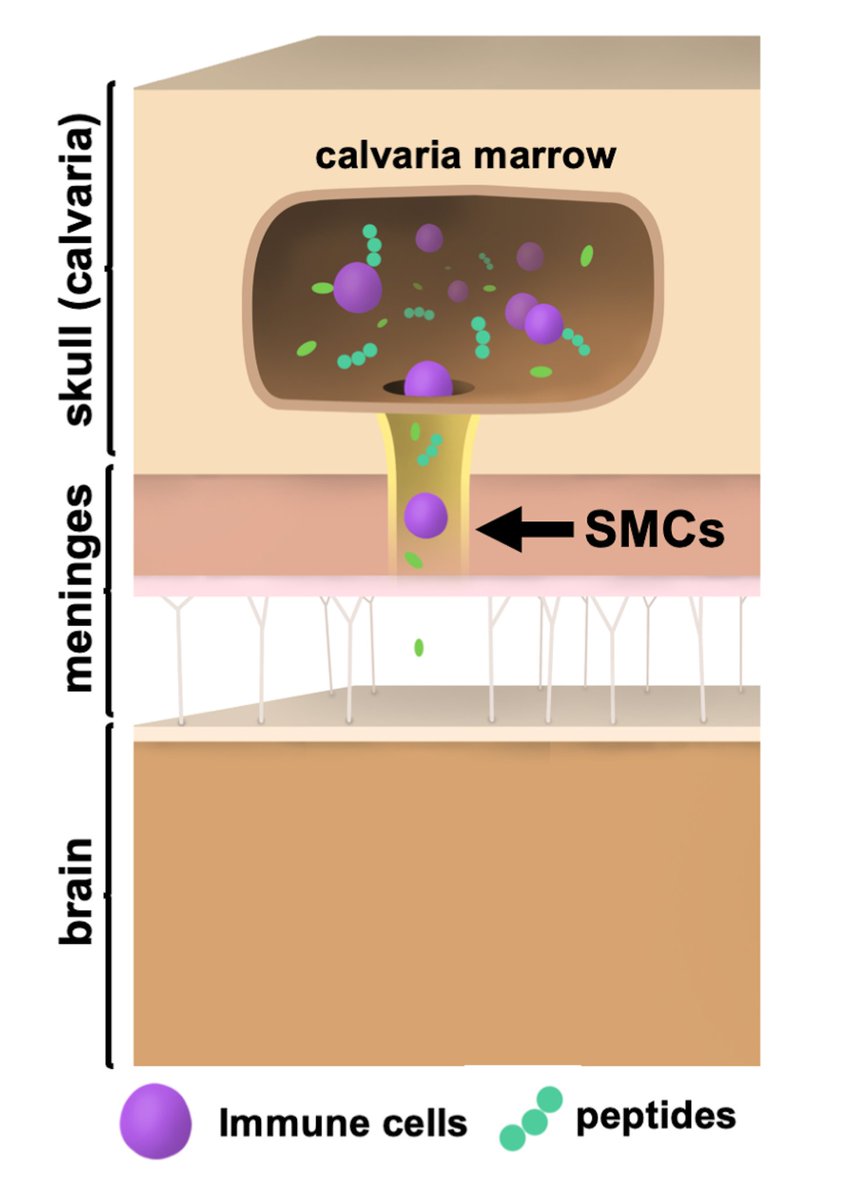
Main Finding 1: Along with many organs, we discovered spike accumulations in the skull marrow niches and recently discovered skull-meninges connections (SMCs), revealing a new route of pathogens into the brain 

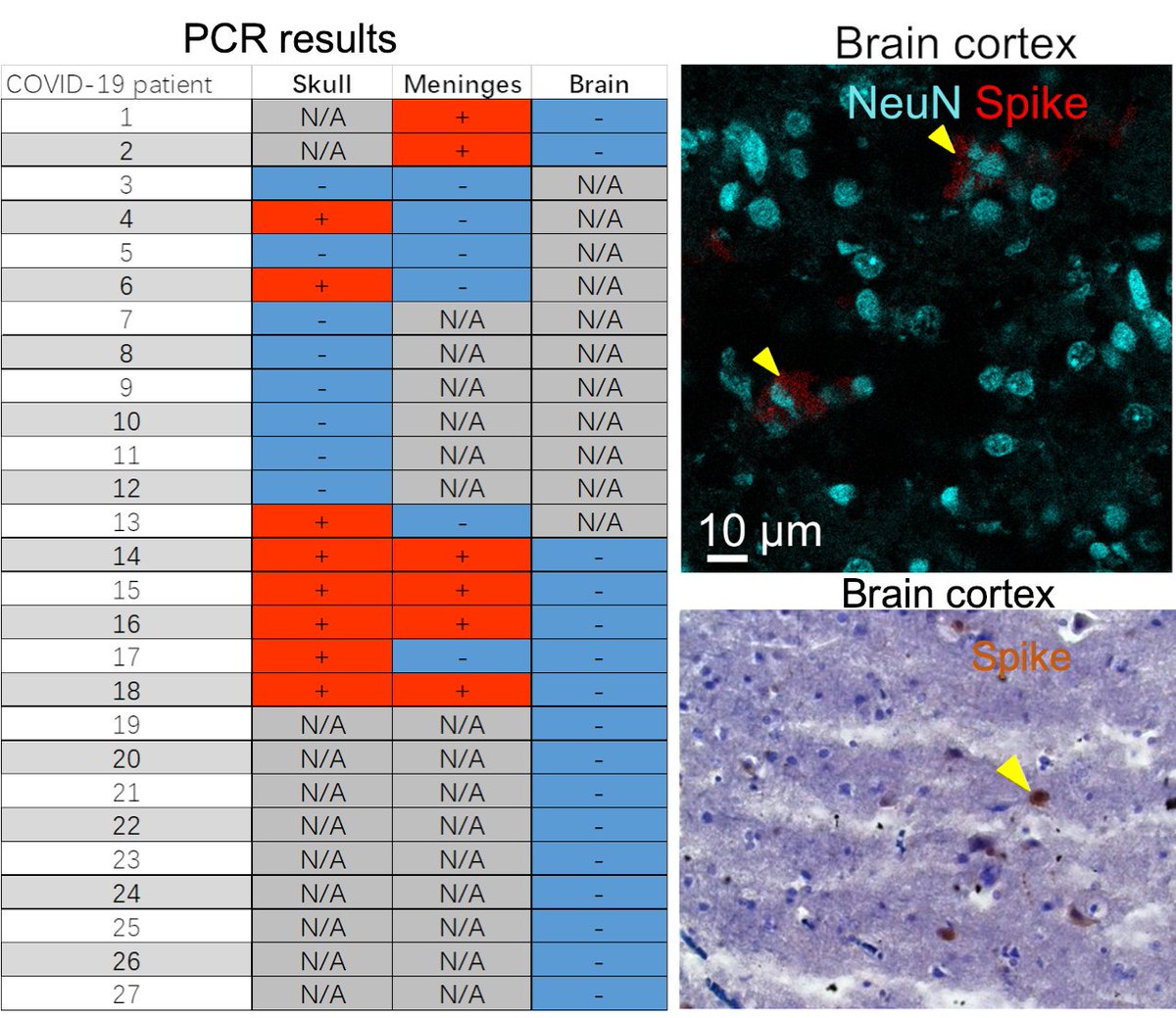
Main Finding 2: Critically, we found the spike protein also in the skull bone marrow niches, and meninges of people who died from COVID-19. 
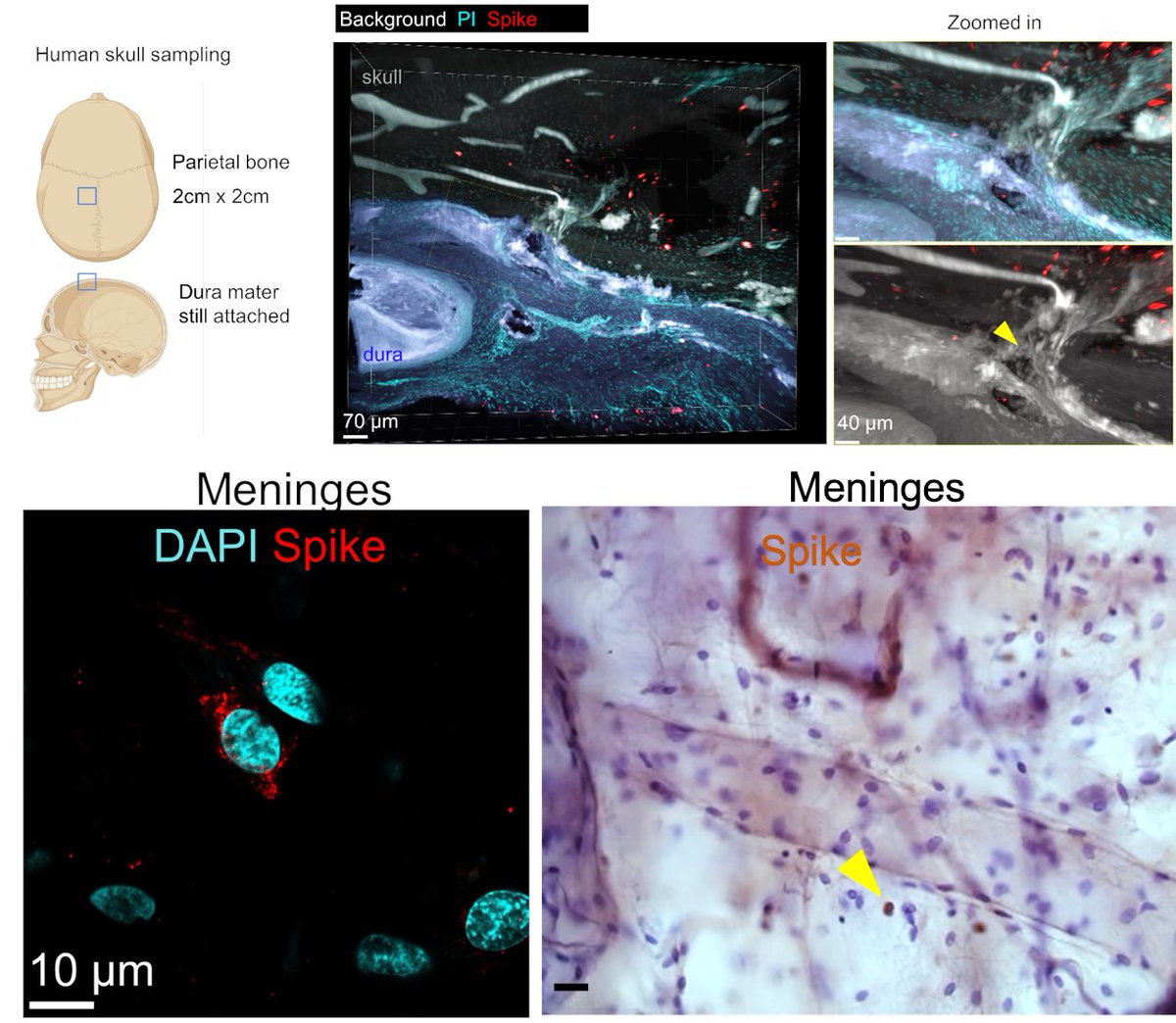
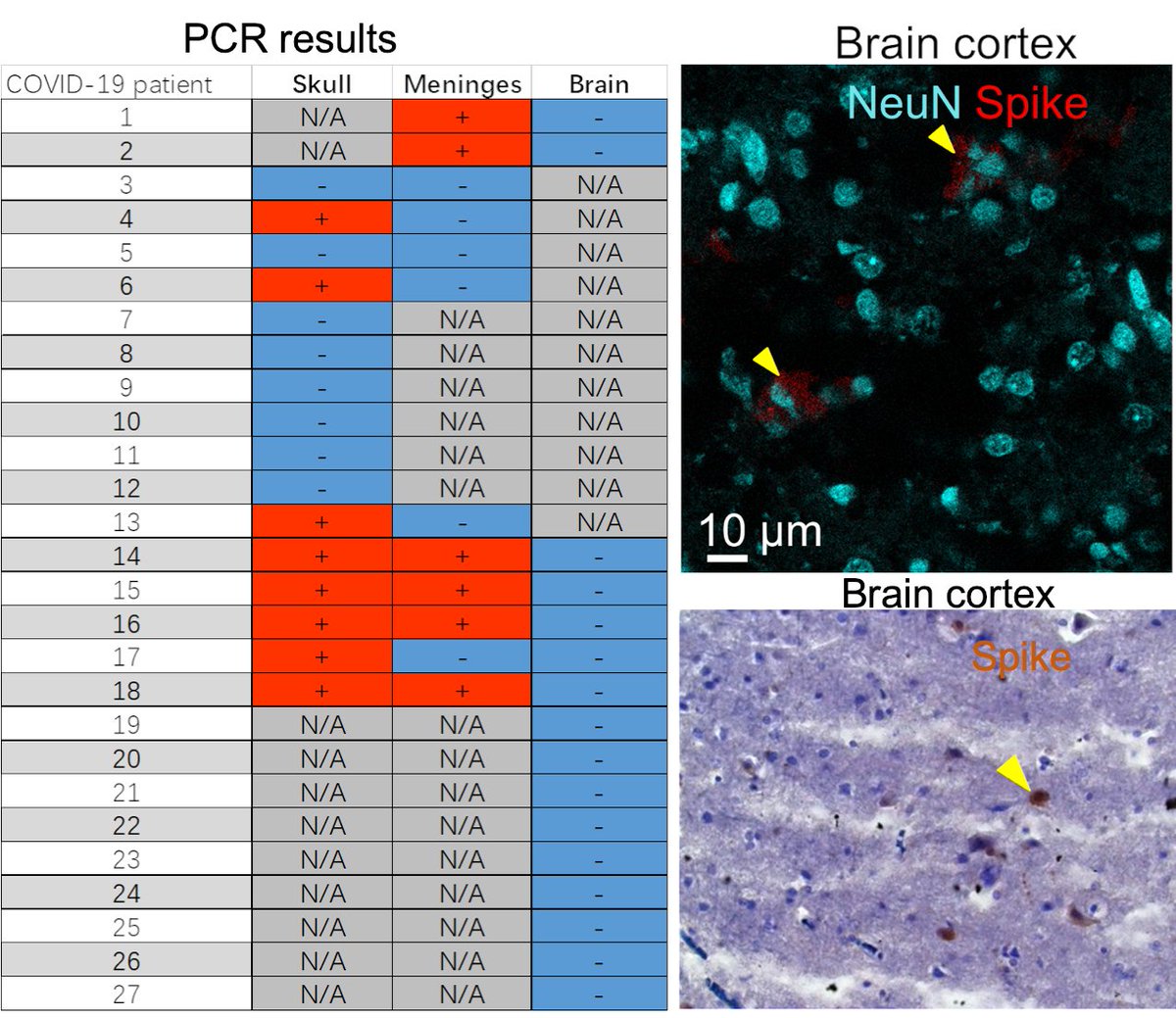
Main Finding 3: Although COVID-19 patients' brain tissue was PCR-negative, spike protein was present in the brain, suggesting a longer half-life compared to viral particles. 
Main Finding 4: Using unbiased proteomics, we found several dysregulated proteins involved in the neurodegeneration, coagulation cascades, neutrophil degranulation, and the PI3K-AKT signaling in the skull marrow, meninges, and brain of COVID-19 patients. 
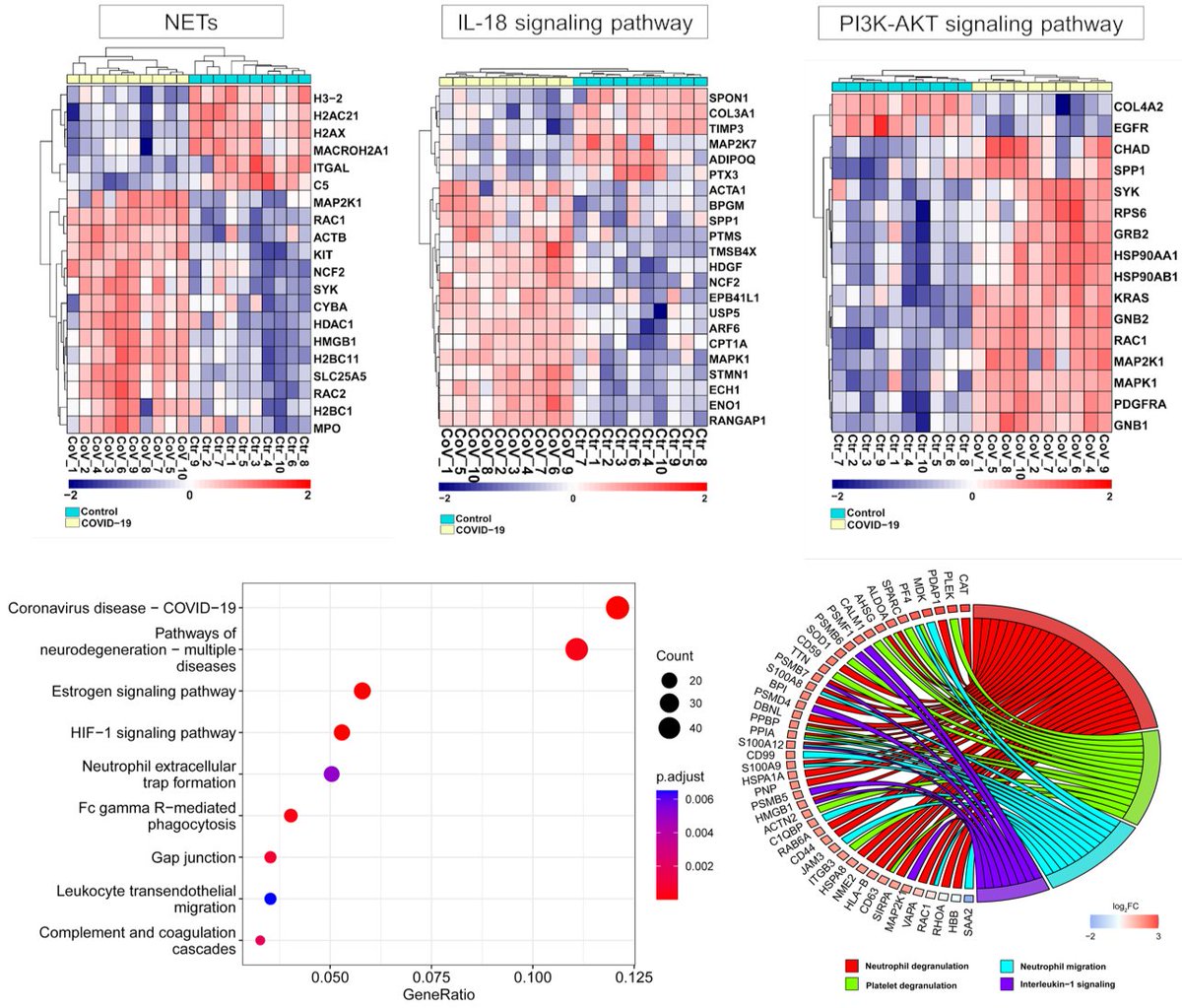
Main Finding 5: SARS-CoV-2 spike S1 protein i.v. injection alone was enough to trigger a wide range of proteomic changes in the skull marrow, meninges, and brain compared to the HA protein. These changes are similar to those observed in virus-infected human samples. 
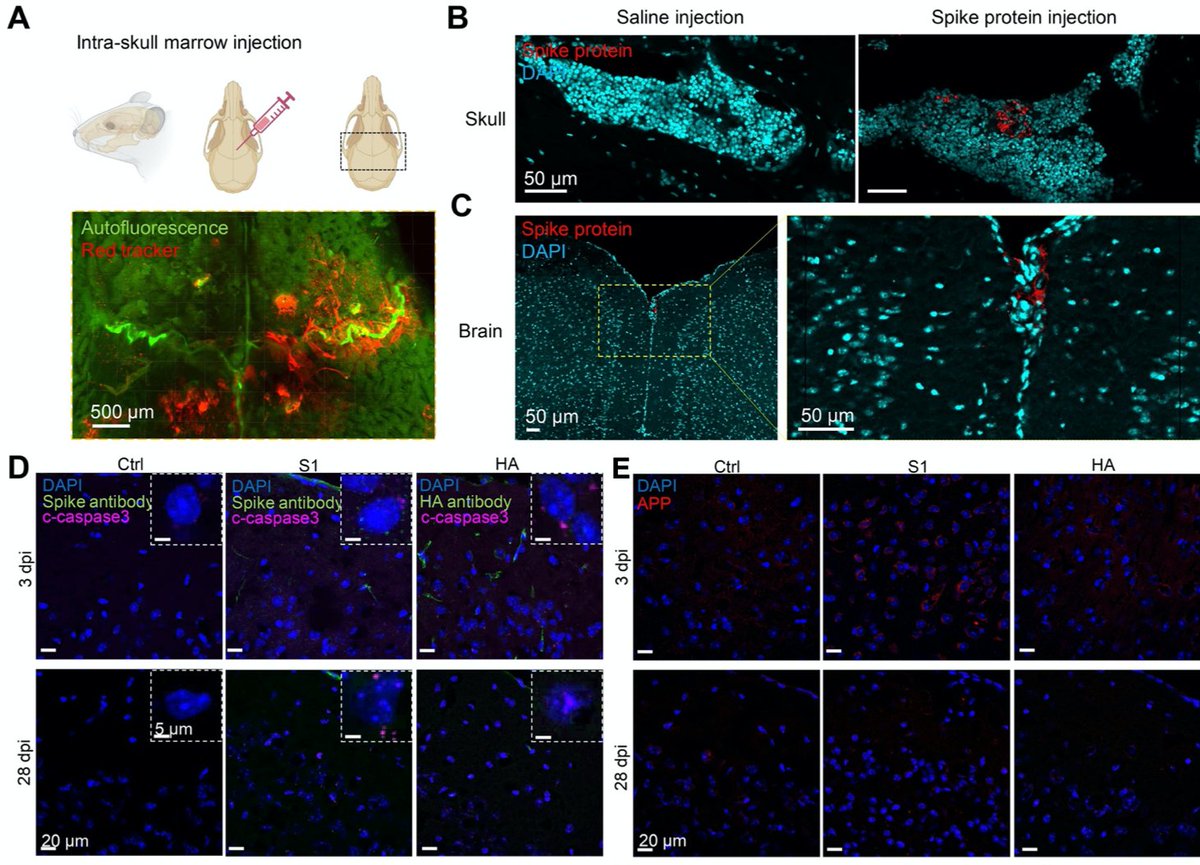
Main Finding 6: Furthermore, injection of spike protein directly into skull marrow resulted in acute and long-term neuronal injury in mouse brain cortex tissue (observed as cell death and increased APP expression), while influenza HA did not lead to any changes. 
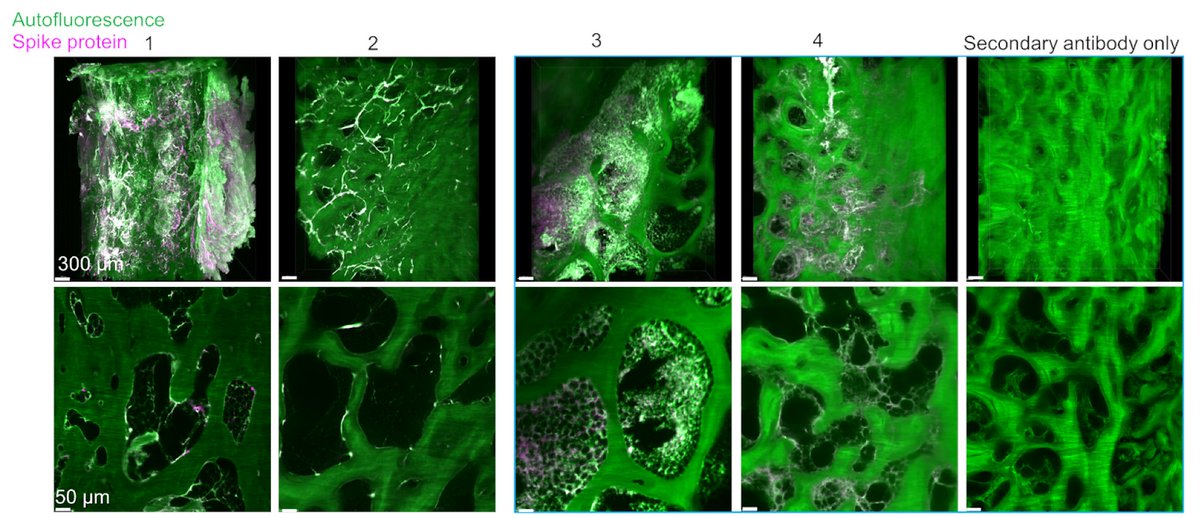
Main Finding 7: Strikingly, we found spike accumulation in ~60% of people who had COVID-19 in the past long after their recovery. Thus, the identified spike in the human skull beyond the viral detection time might be a co-factor in developing long-term COVID-19 symptoms. 
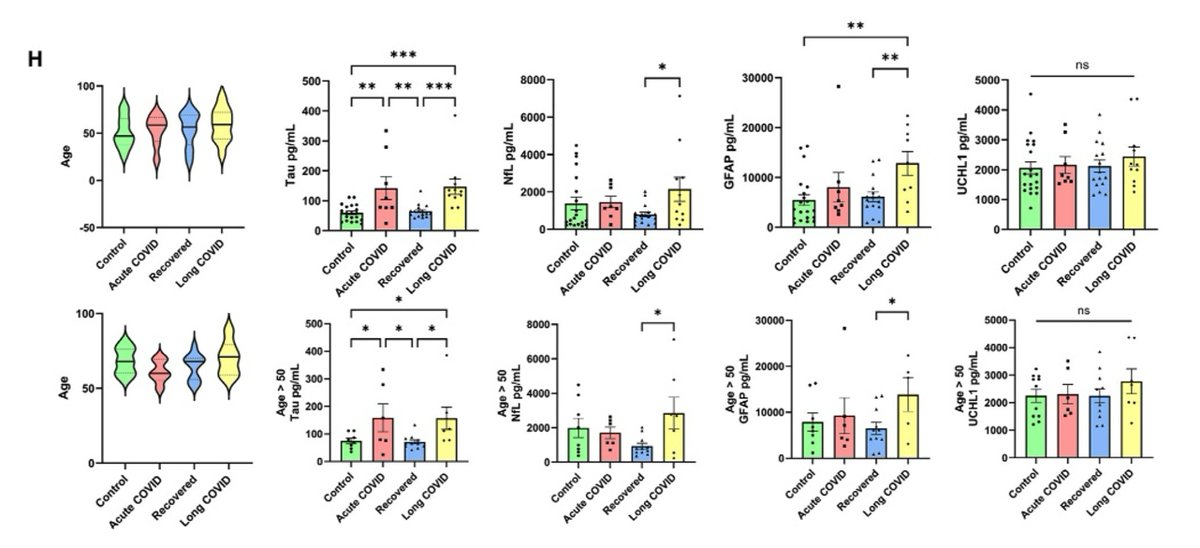
Main Finding 8: Compared to the control group, patients with Long COVID showed significantly elevated levels of neurodegenerative disease-related proteins, such as Tau protein and neurofilament light chain (NfL), in their cerebrospinal fluid. 
Main Finding 9: The immune response triggered by the spike protein may activate the MAPK-JNK signaling pathway, leading to neuronal stress and inflammation. 
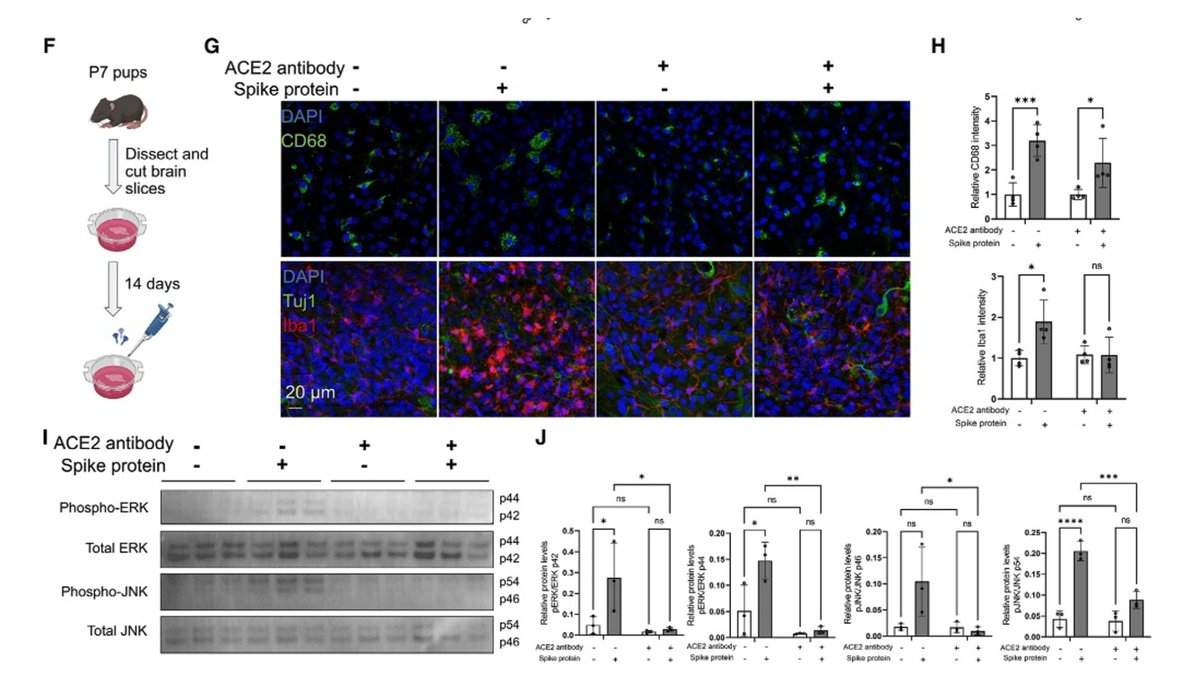
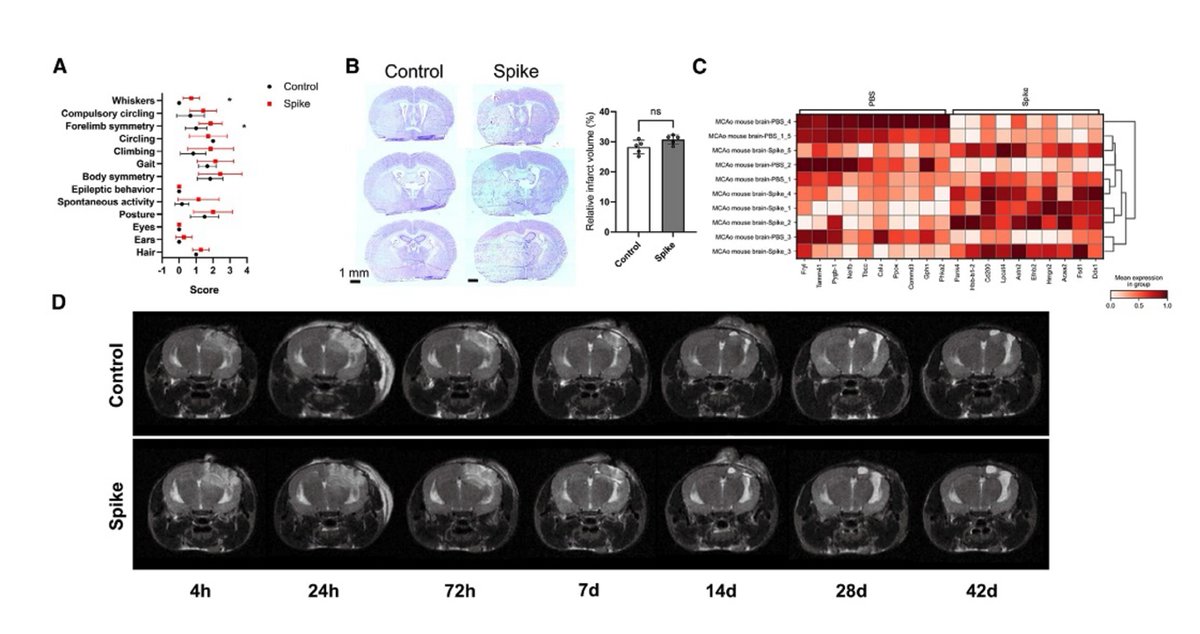
Main Finding 10: In stroke and traumatic brain injury mouse models, the presence of spike protein exacerbates brain tissue damage, suggesting that it may enhance the susceptibility of the nervous system to further insults. 
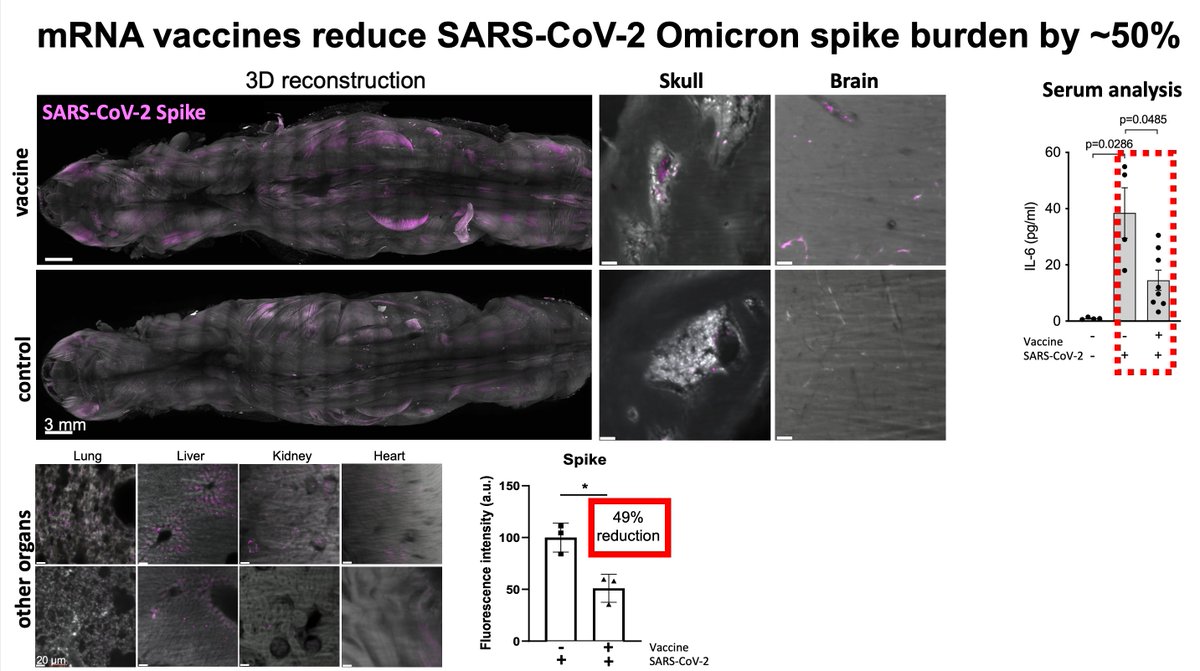
Main Finding 11: The accumulation of spike protein in mice vaccinated with the BioNTech/Pfizer vaccine was significantly reduced, but not completely eliminated. This suggests that vaccination can significantly reduce the long-term effects of the virus on the nervous system, providing important support for reducing the risk of sequelae of COVID-19.
Clinical implications and future research directions
• Diagnosis: Neural injury markers in cerebrospinal fluid may be used as an important indicator for evaluating the sequelae of COVID-19.
• Treatment: In the future, strategies for the removal or inhibition of spike proteins may become an important way of treating the sequelae of COVID-19.
This study on the skull-meninges-brain axis and the persistence of spike proteins offers novel insights into the neural injury mechanism underlying COVID-19 sequelae. Future research should delve deeper into the mechanisms of spike protein entry and retention in brain tissue, as well as potential variations among different COVID-19 variants. Such investigations may pave the way for accurate diagnosis and personalized treatment of COVID-19 neurological sequelae.
• Diagnosis: Neural injury markers in cerebrospinal fluid may be used as an important indicator for evaluating the sequelae of COVID-19.
• Treatment: In the future, strategies for the removal or inhibition of spike proteins may become an important way of treating the sequelae of COVID-19.
This study on the skull-meninges-brain axis and the persistence of spike proteins offers novel insights into the neural injury mechanism underlying COVID-19 sequelae. Future research should delve deeper into the mechanisms of spike protein entry and retention in brain tissue, as well as potential variations among different COVID-19 variants. Such investigations may pave the way for accurate diagnosis and personalized treatment of COVID-19 neurological sequelae.
Thanks to all contributors @PuellesVictor @janczogalla @
@ozumsehnaz @shan_heather
@mayarbali @ilginkolabas @SelinUlukaya @IzabelaHorvath
@NatalieKrahmer @AlionderCPC @Tobias_B_Huber @IngoBechmann @UlrikeProtzer @HarsharanBhatia @Farida80168644 Work done at the @HelmholtzMunich,, @LMU_Muenchen, @LMU_Uniklinikum, @ISD_Research, @TU_Muenchen, supported by @SyNergy_Cluster, @ERC_Research, @NOMIS and graduate programs @IMPRS_LS, GSN-LMU, and MMRS-LMU.
@ozumsehnaz @shan_heather
@mayarbali @ilginkolabas @SelinUlukaya @IzabelaHorvath
@NatalieKrahmer @AlionderCPC @Tobias_B_Huber @IngoBechmann @UlrikeProtzer @HarsharanBhatia @Farida80168644 Work done at the @HelmholtzMunich,, @LMU_Muenchen, @LMU_Uniklinikum, @ISD_Research, @TU_Muenchen, supported by @SyNergy_Cluster, @ERC_Research, @NOMIS and graduate programs @IMPRS_LS, GSN-LMU, and MMRS-LMU.
Thanks to all contributors @PuellesVictor @janczogalla @Inderjeetbph @d_jeridi @ozumsehnaz @shan_heather
@mayarbali @ilginkolabas @SelinUlukaya @IzabelaHorvath @NatalieKrahmer @AlionderCPC @SiegfriedU @Tobias_B_Huber @GunterHoglinger @IngoBechmann @UlrikeProtzer @MarkusElsner1 @HarsharanBhatia @Farida80168644
Work done at the @HelmholtzMunich, @LMU_Muenchen, @LMU_Uniklinikum, @ISD_Research, @TU_Muenchen, supported by @SyNergy_Cluster, @ERC_Research, @NOMIS and graduate programs @IMPRS_LS, GSN-LMU, and MMRS-LMU.
@mayarbali @ilginkolabas @SelinUlukaya @IzabelaHorvath @NatalieKrahmer @AlionderCPC @SiegfriedU @Tobias_B_Huber @GunterHoglinger @IngoBechmann @UlrikeProtzer @MarkusElsner1 @HarsharanBhatia @Farida80168644
Work done at the @HelmholtzMunich, @LMU_Muenchen, @LMU_Uniklinikum, @ISD_Research, @TU_Muenchen, supported by @SyNergy_Cluster, @ERC_Research, @NOMIS and graduate programs @IMPRS_LS, GSN-LMU, and MMRS-LMU.
You can read the news from our research center @HelmholtzMunich on our study:
https://x.com/HelmholtzMunich/status/1862528702531166470
News from our University LMU Munich:
https://x.com/LMU_Muenchen/status/1863859545988018385
a sketch on our work:
https://x.com/ImmunoSketch/status/1863858963260825889
• • •
Missing some Tweet in this thread? You can try to
force a refresh