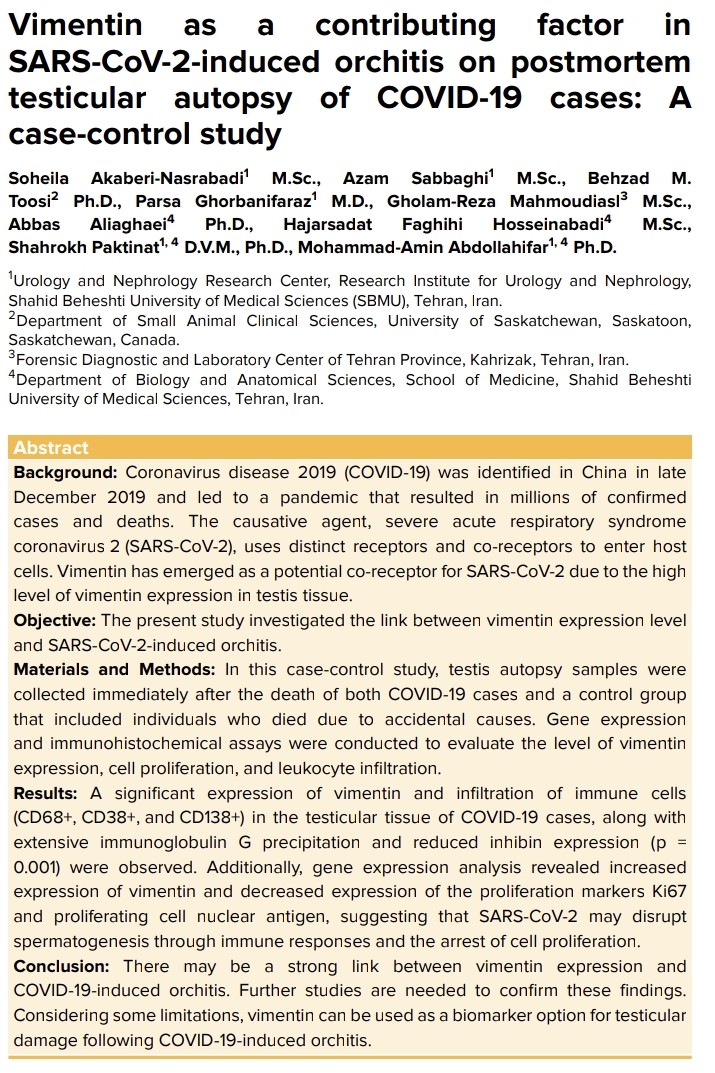
When SARS-CoV-2 INFECTION can facilitate an ATTACK on our BRAIN, by PRIONS and AMYLOIDS.
(🧵mega-thread 8 studies)
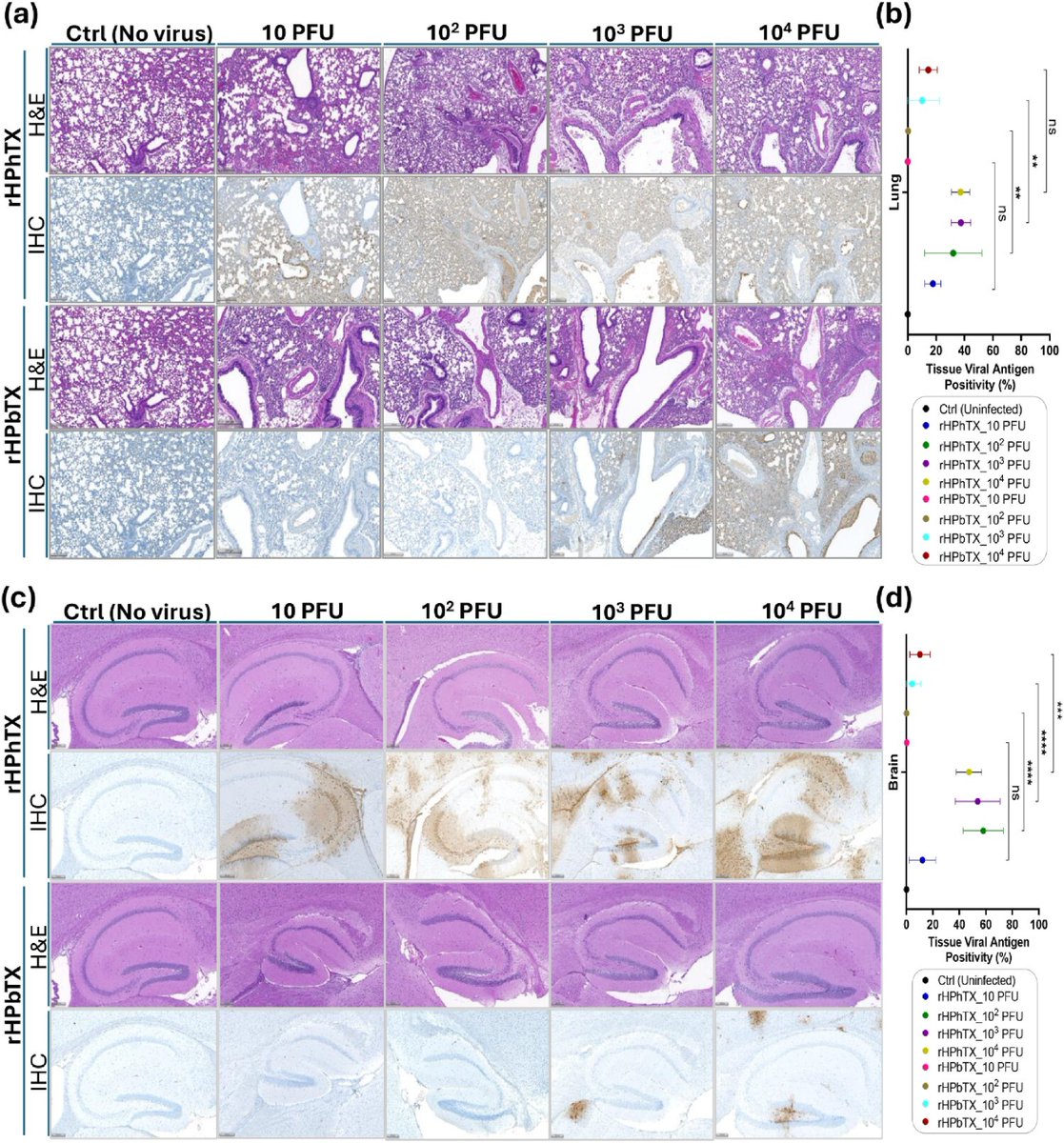
Recent studies have illuminated a concerning aspect of SARS-CoV-2 infections: the potential impact on the brain through the involvement of prions and amyloids.
(🧵mega-thread 8 studies)
Recent studies have illuminated a concerning aspect of SARS-CoV-2 infections: the potential impact on the brain through the involvement of prions and amyloids.
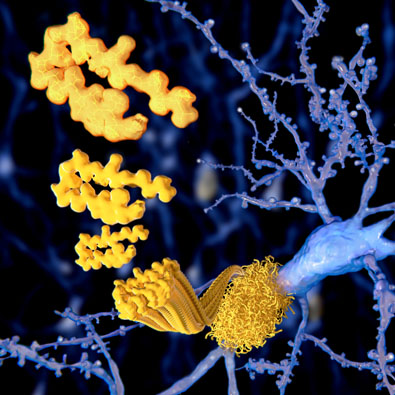
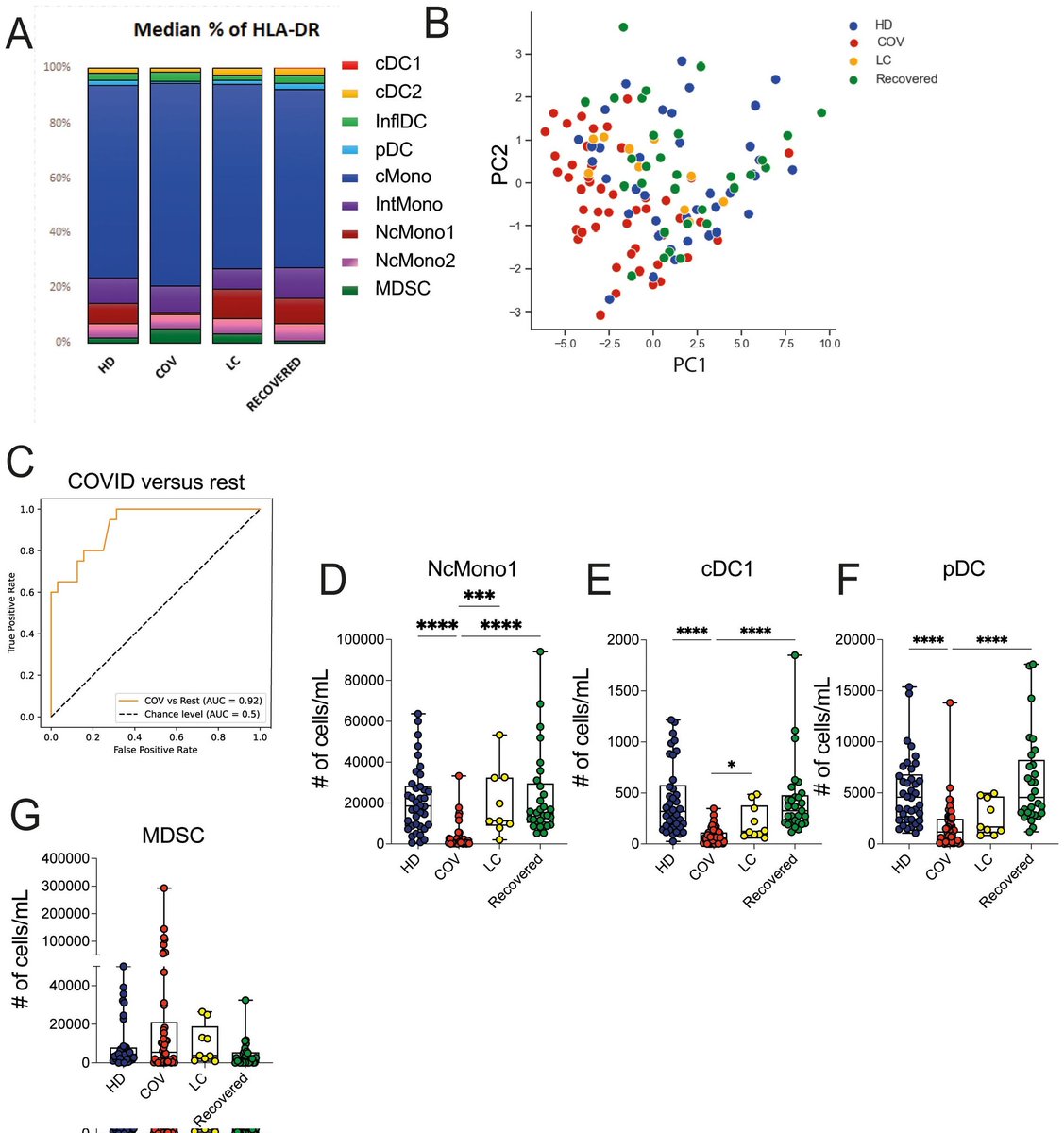
2) As the virus enters the body, it can trigger pathological processes that lead to the misfolding of proteins, resulting in the formation of amyloid plaques and prion-like aggregates. These abnormal protein structures can disrupt neural function ... 
3) ... contributing to neurological symptoms such as cognitive decline, memory loss, and other forms of encephalopathy. The interplay between infection and these neurodegenerative mechanisms underscores the complexity of COVID-19 and its long-term consequences on brain health. 
12) Thanks for reading 🙏
https://x.com/ejustin46/status/1784448925123916223?t=zETpKe066hhv8sbxYdwgVA&s=19
• • •
Missing some Tweet in this thread? You can try to
force a refresh