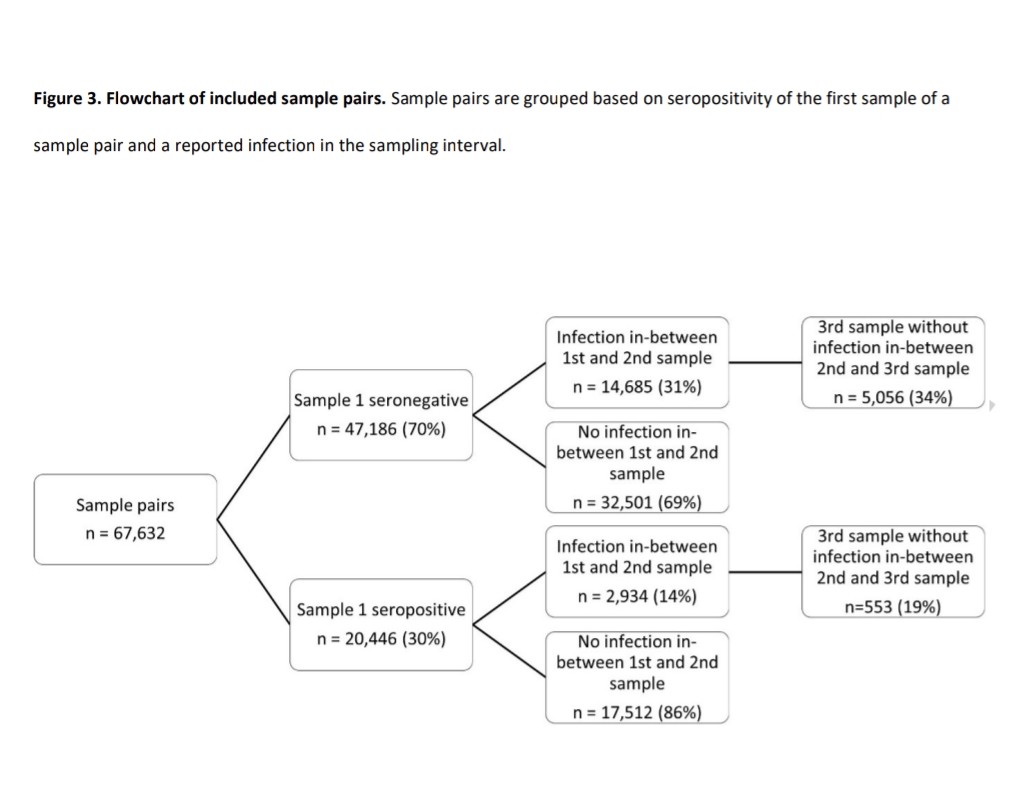
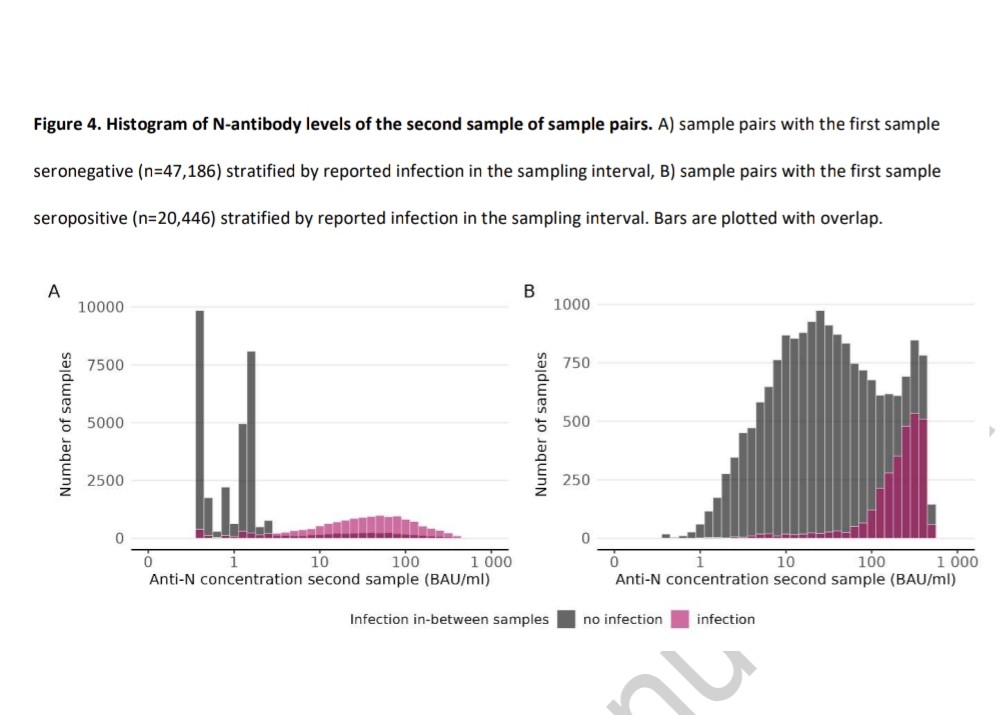
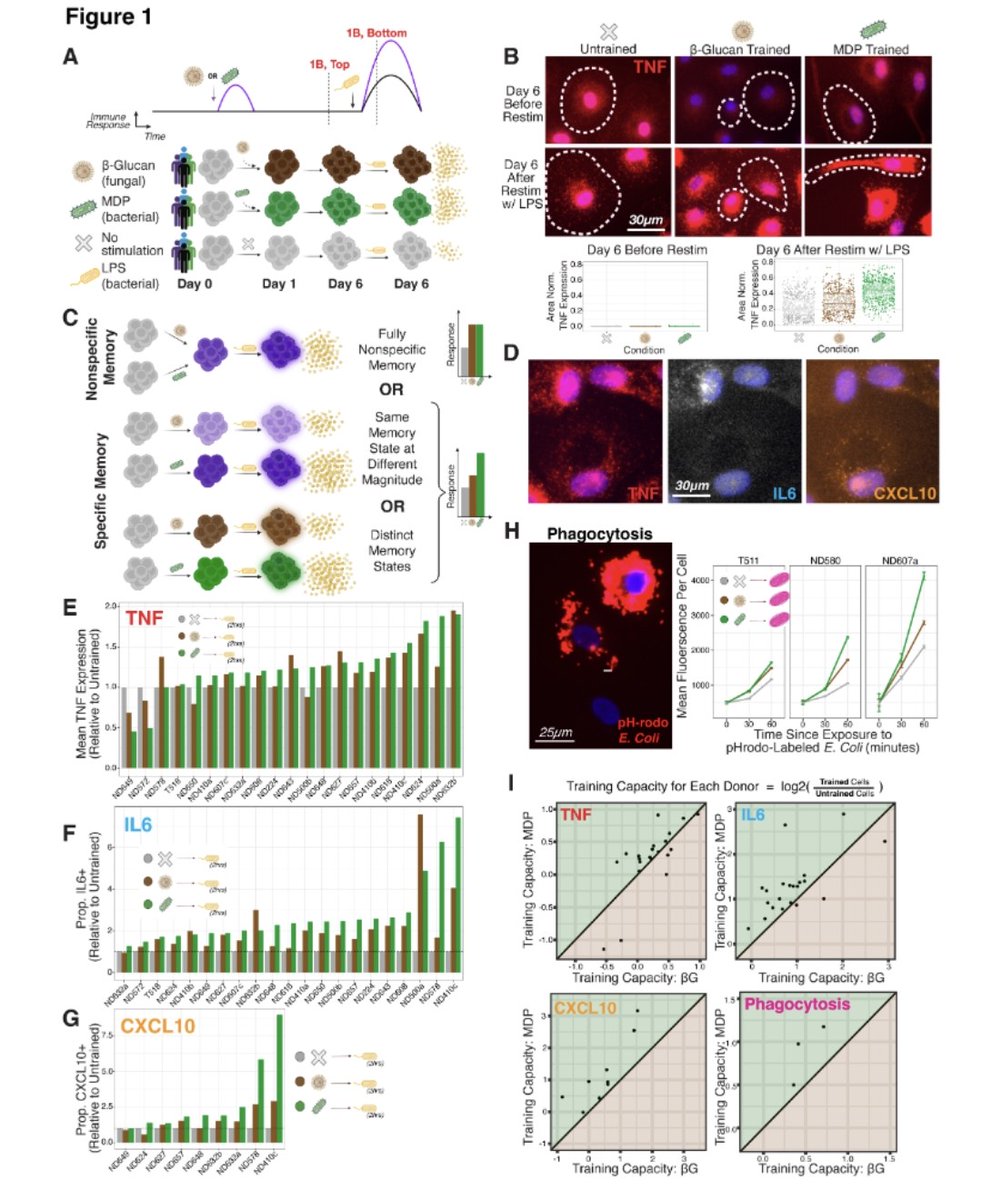
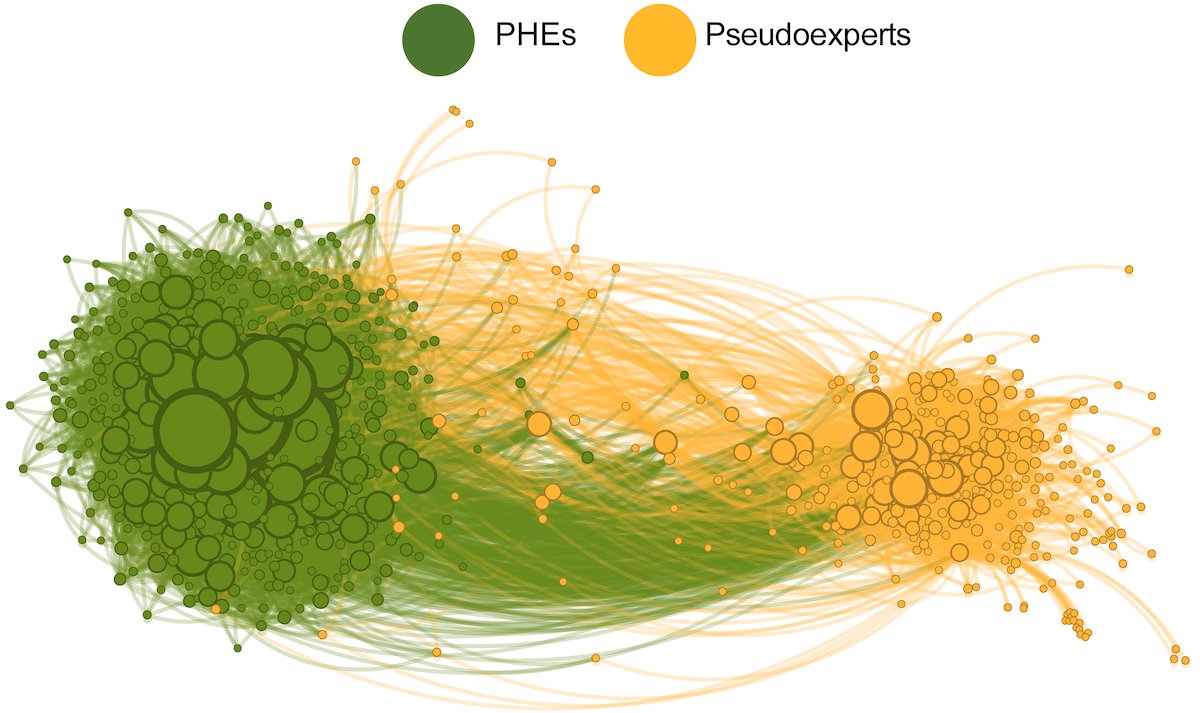
The OMICRON EFFECT:
How Variants Mutations Reshaped the COVID-19 Immunity Landscape 💥💯
nature.com/articles/s4158…
How Variants Mutations Reshaped the COVID-19 Immunity Landscape 💥💯
nature.com/articles/s4158…

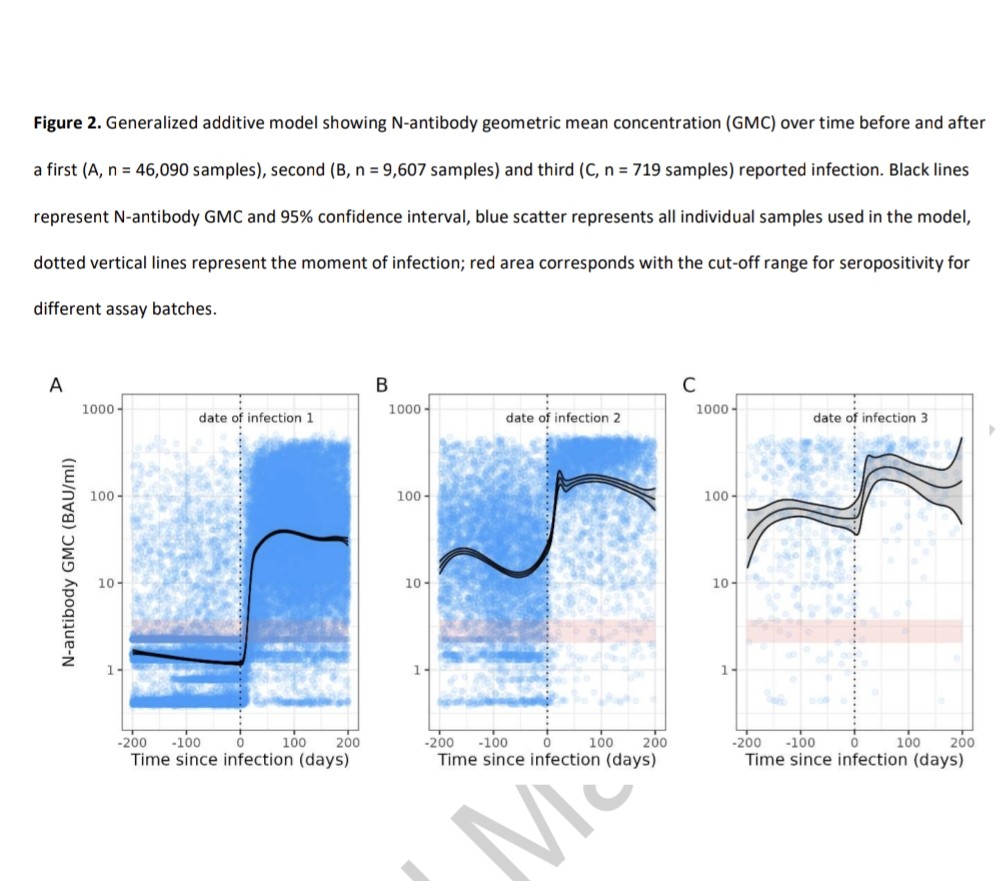
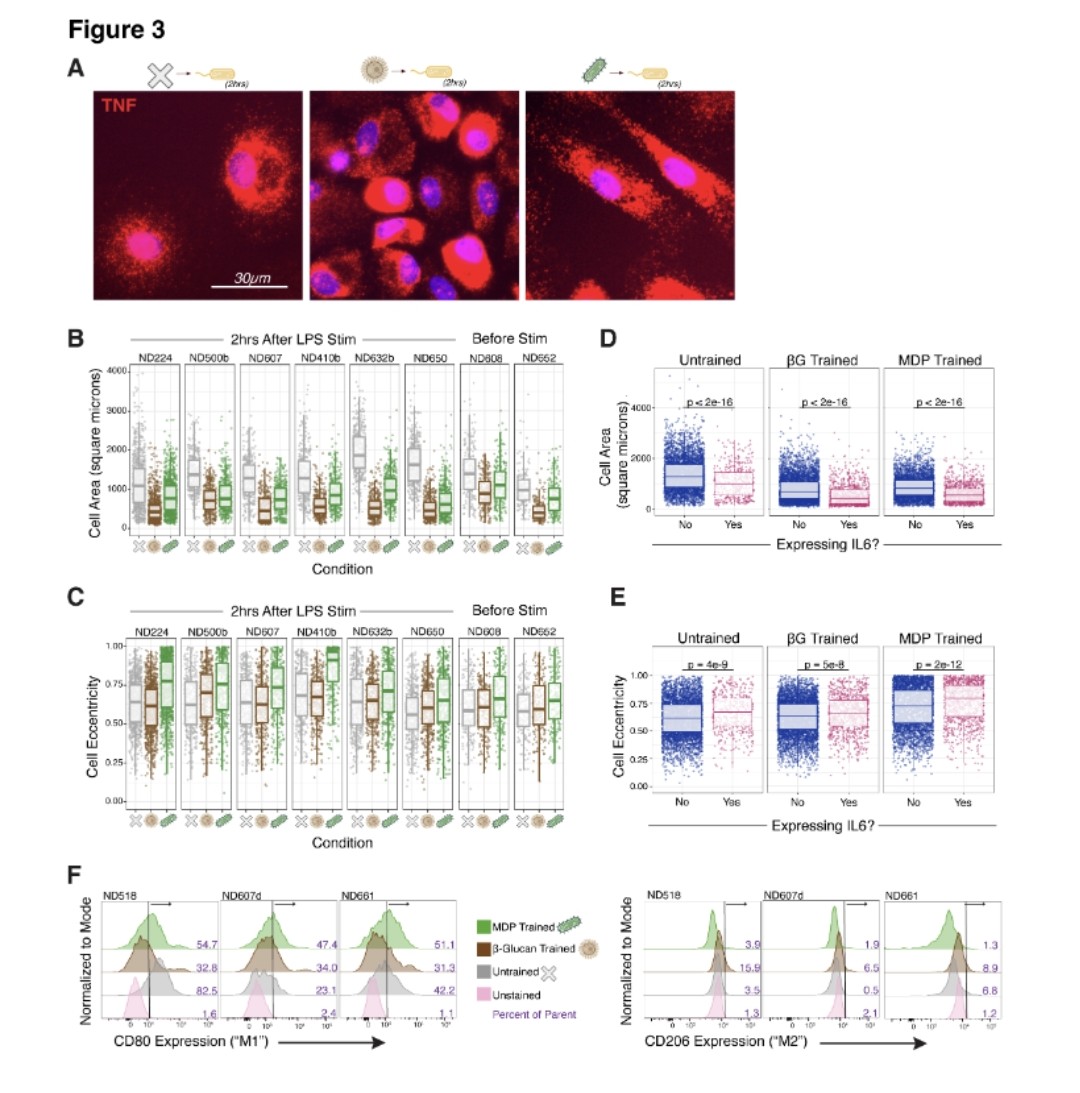
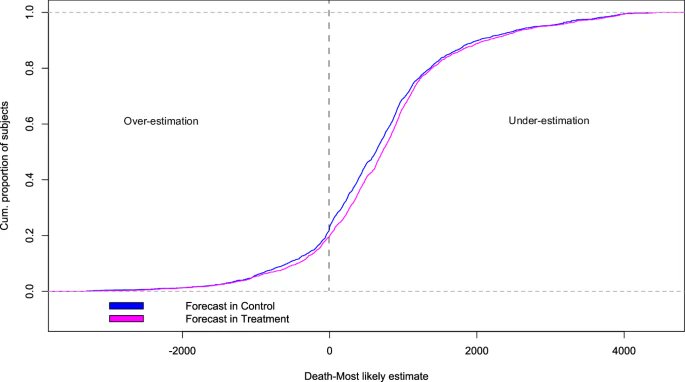
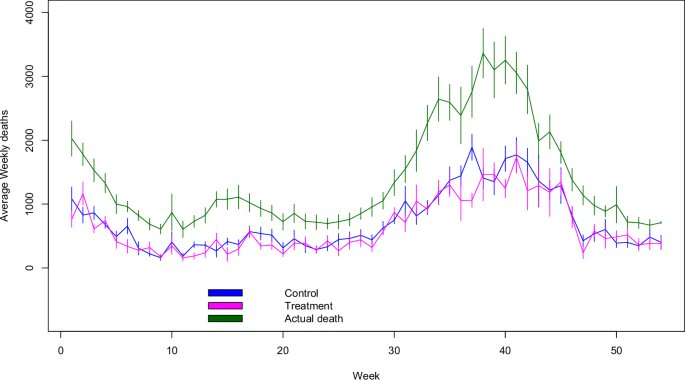
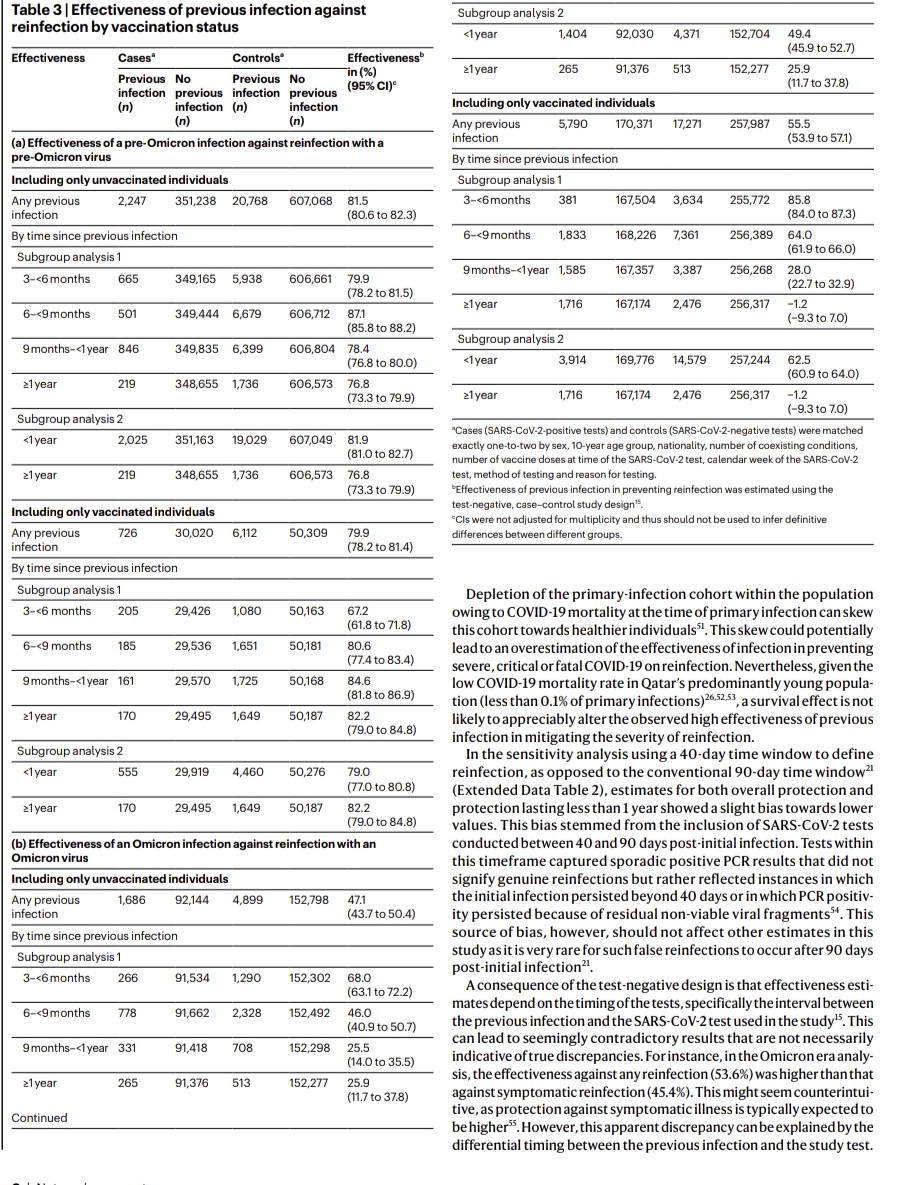
2) COVID-19 saw rapid SARS-CoV-2 evolution. Pre-Omicron, prior infection provided strong, lasting protection against reinfection. But Omicron changed this - recent Omicron infections only briefly protected, with protection waning quickly. 
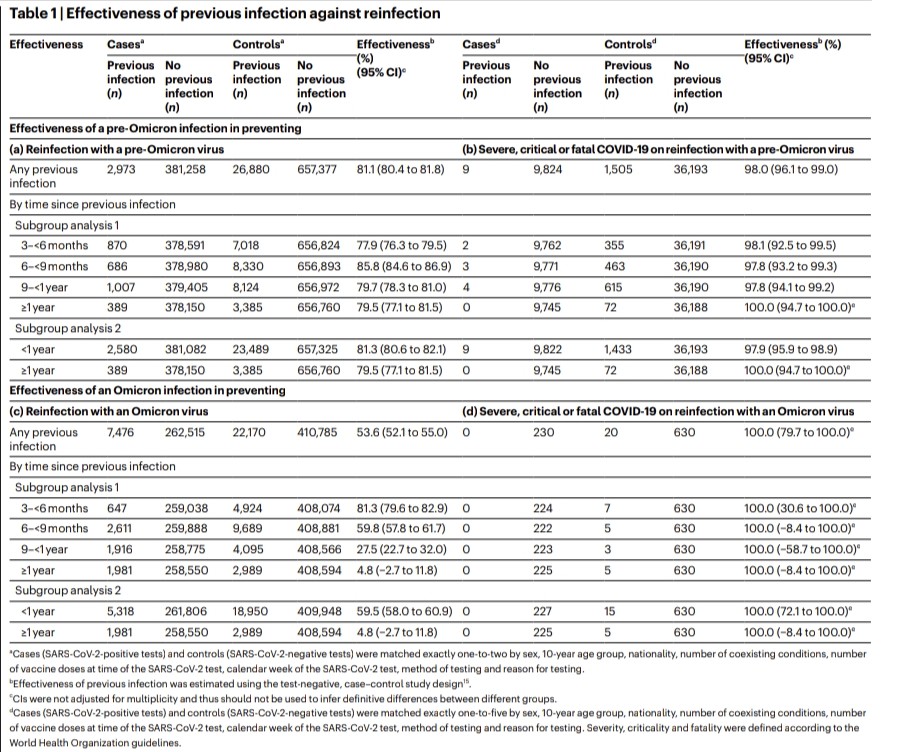
3) Researchers link this to a shift in evolutionary pressures. Initially, the virus adapted to spread more easily. But with Omicron, when many had immunity, it evolved to evade that immunity through mutations, especially in the spike protein. 
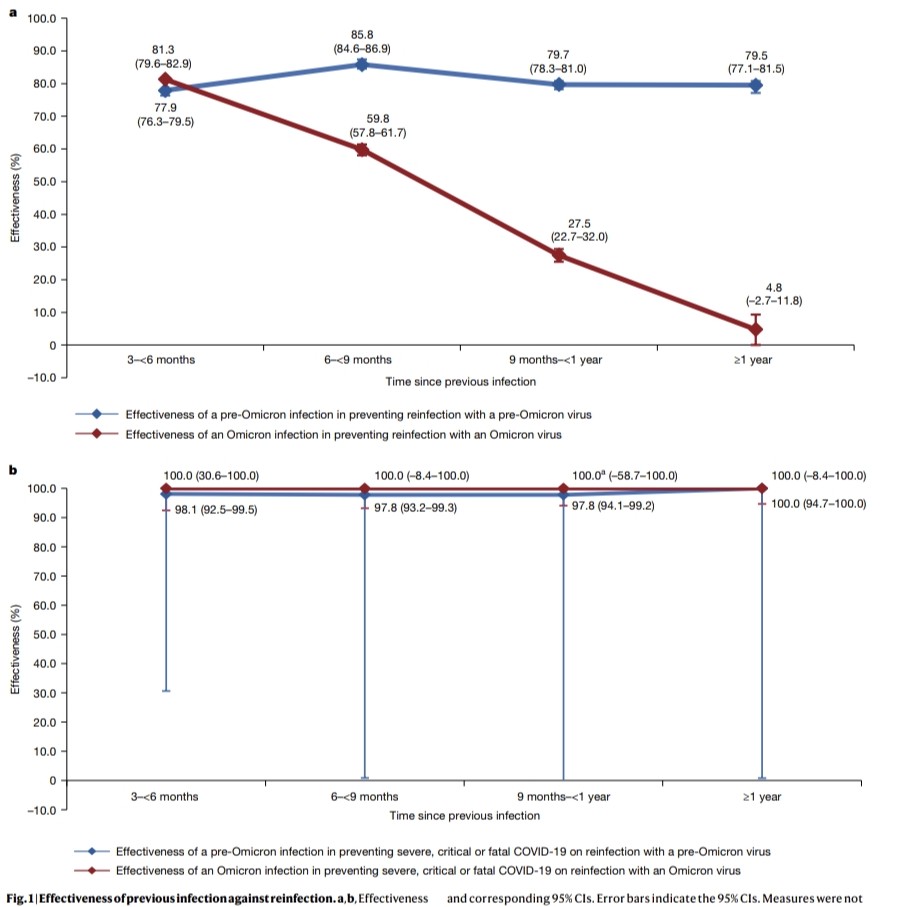
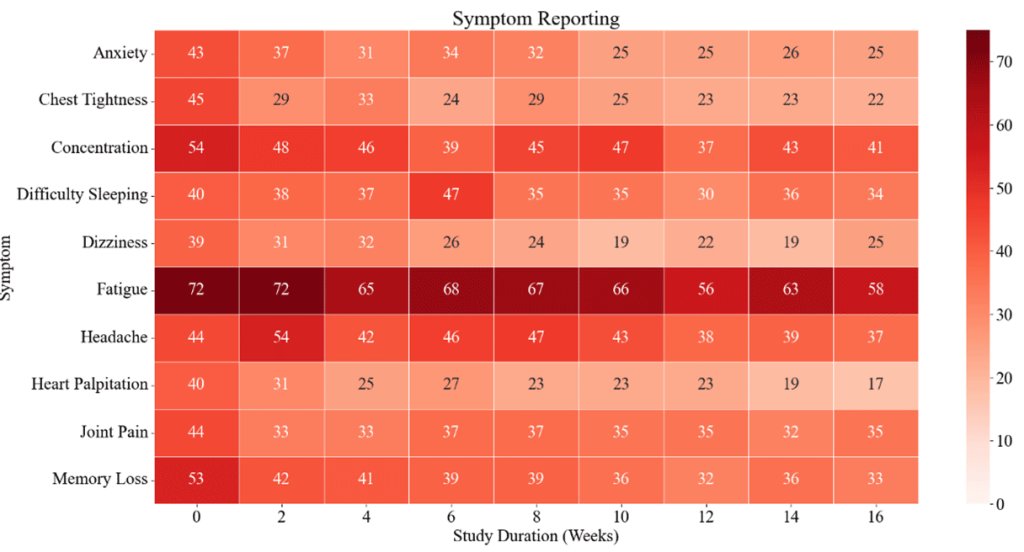
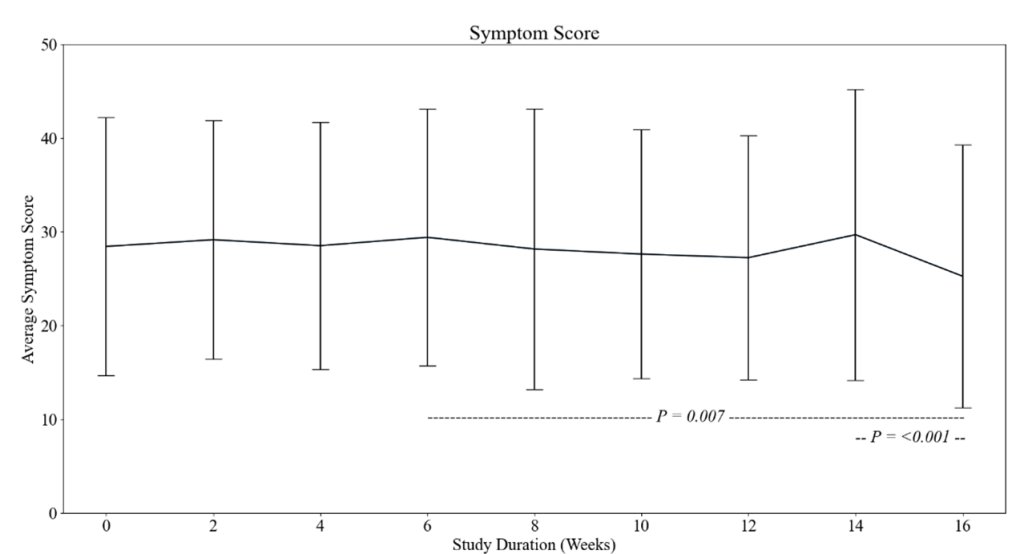
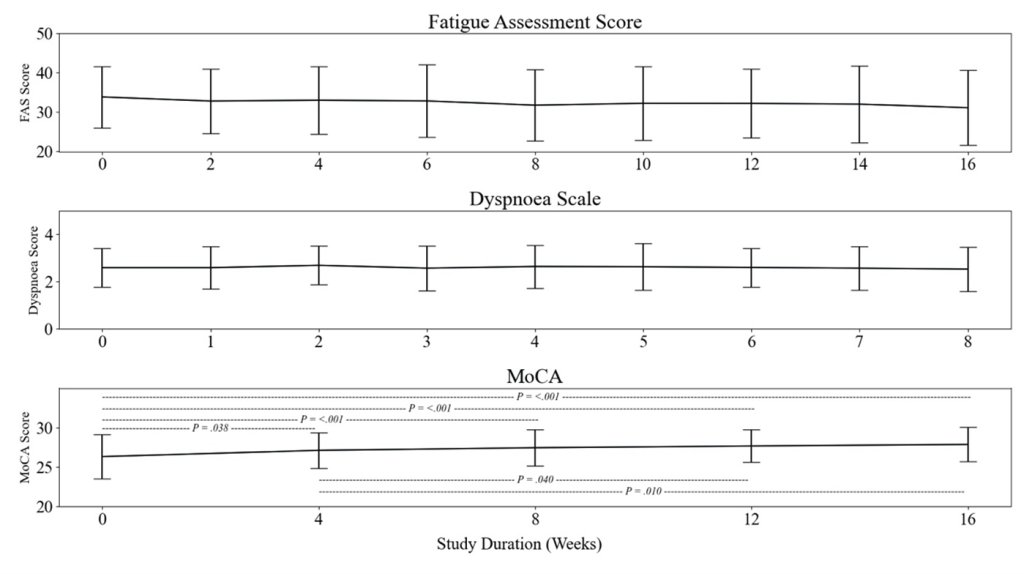
4) This immune evasion explains Omicron's lack of lasting protection. Yet prior infection still strongly prevented severe disease, suggesting different immune responses protect against mild vs. severe illness. 
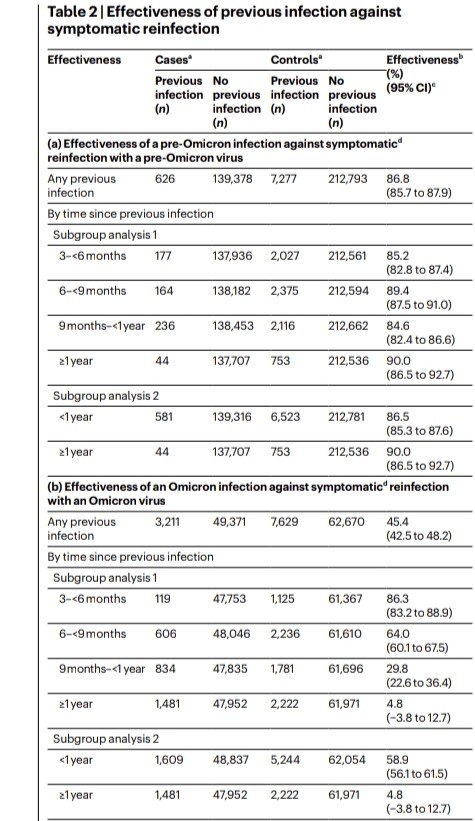
5) This highlights SARS-CoV-2's adaptability and the need to continually monitor the virus and update vaccines to keep up with its changes.
Thanks for reading 🙏
Thanks for reading 🙏
• • •
Missing some Tweet in this thread? You can try to
force a refresh