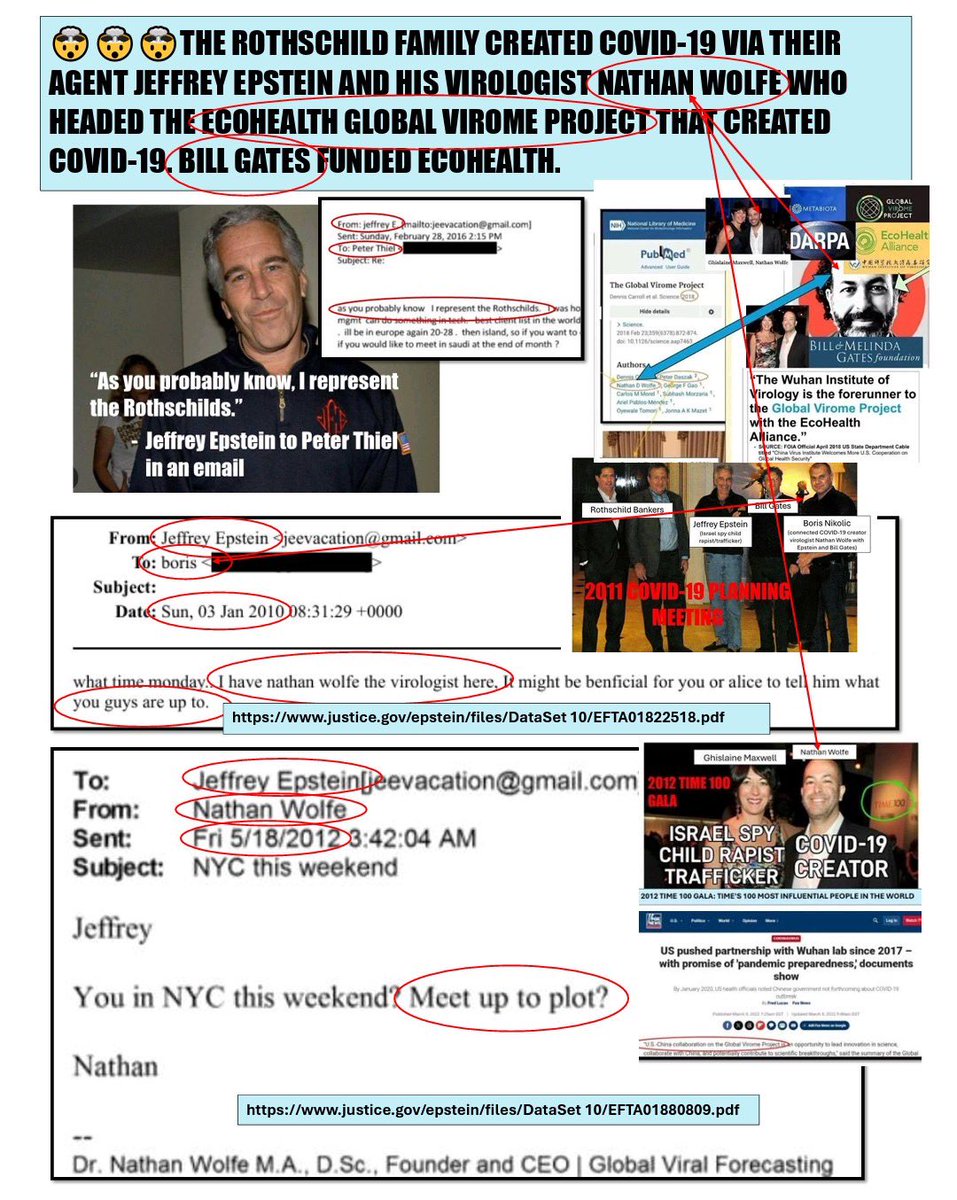
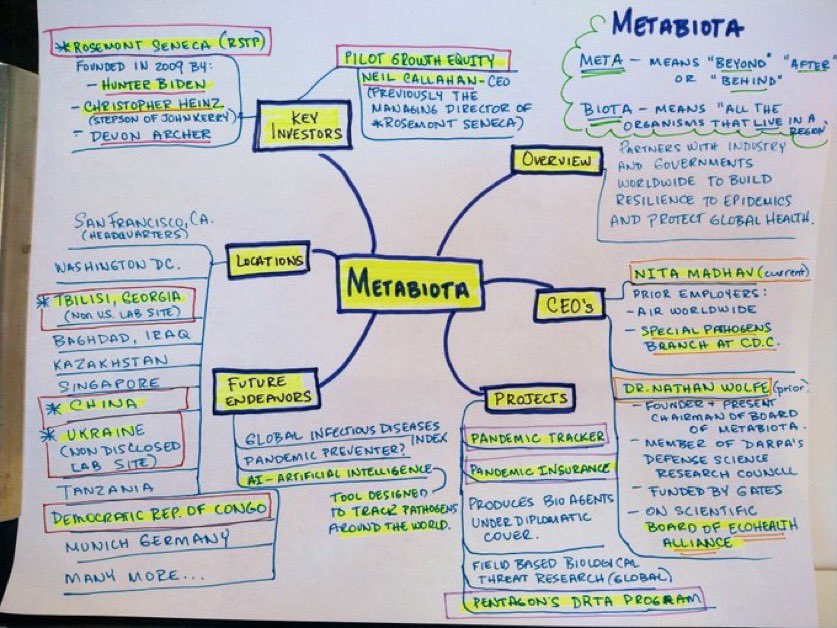
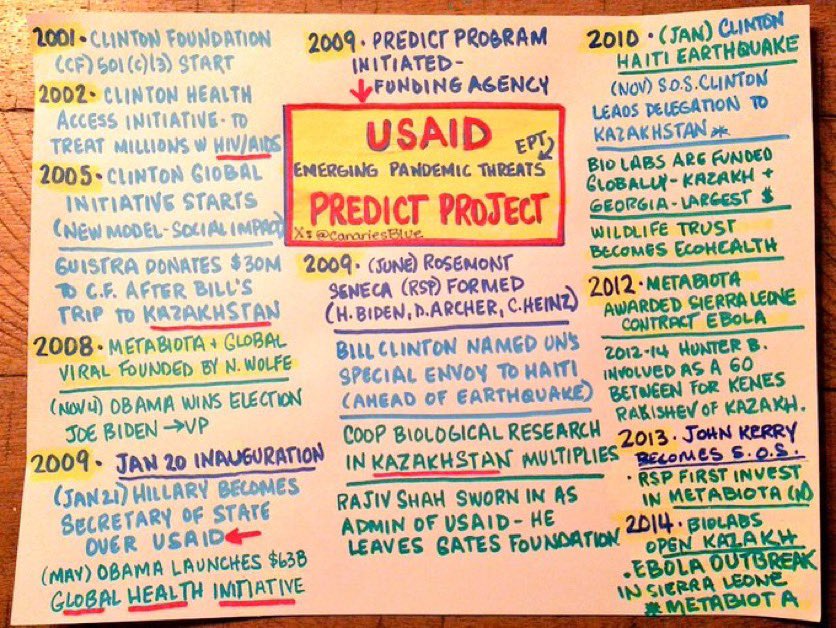
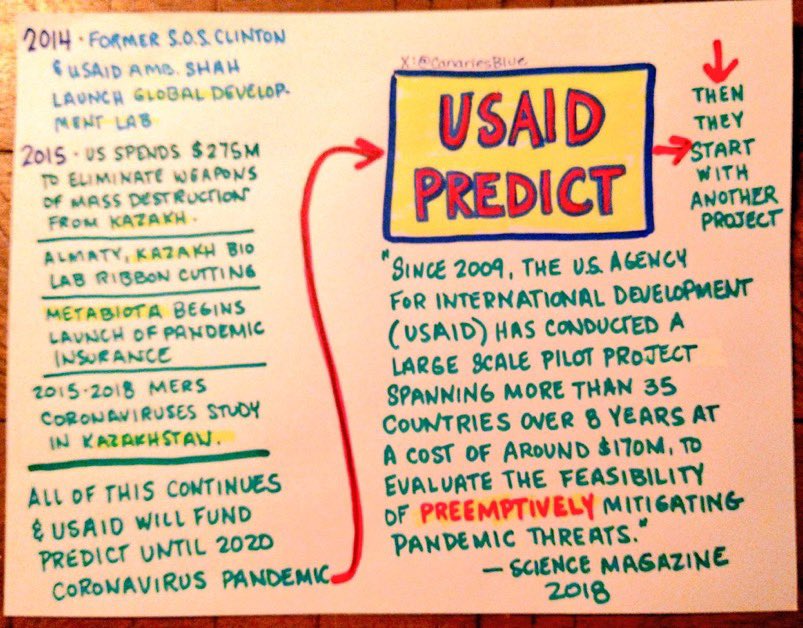
So the Yale Listen Study said 709 days per their testing of vaxinjured still having continuous spike production (which I am a participant in this study) and show T cell exhaustion etc
Well it’s actually longer than that:
My first injection was 12/23/2020 and I haven’t stopped producing spike and it’s now 02/21/2025
8 months after my 2 Moderna injections I still had large amounts of spike detected per antigen testing- & I’ve never had Covid( results below)
That’s 1,521 days - of continuous spike protein production with NO OFF SWITCH since my first injection & STILL in my body to date
Let that sink in!
(3 years worth of Cytokine 14 panels & S1 immune subset panels below)
I am a documented, confirmed & diagnoses Adverse reaction to MRNA vaccine ( T50.B95A)


Well it’s actually longer than that:
My first injection was 12/23/2020 and I haven’t stopped producing spike and it’s now 02/21/2025
8 months after my 2 Moderna injections I still had large amounts of spike detected per antigen testing- & I’ve never had Covid( results below)
That’s 1,521 days - of continuous spike protein production with NO OFF SWITCH since my first injection & STILL in my body to date
Let that sink in!
(3 years worth of Cytokine 14 panels & S1 immune subset panels below)
I am a documented, confirmed & diagnoses Adverse reaction to MRNA vaccine ( T50.B95A)



@threadreaderapp unroll
• • •
Missing some Tweet in this thread? You can try to
force a refresh