Approximately 1 in 9 U.S. children have been diagnosed with ADHD, yet traditional stimulant medications often fall short in efficacy and tolerability. (CHADD, 2021)
Recent advancements have introduced novel therapies targeting multiple neurotransmitter systems, expanding treatment options and potentially improving outcomes. (Neurolaunch, 2023)
Here are 4 emerging treatments that could improve ADHD management by offering alternatives to stimulants and addressing broader neurochemical pathways 🧵👇
Recent advancements have introduced novel therapies targeting multiple neurotransmitter systems, expanding treatment options and potentially improving outcomes. (Neurolaunch, 2023)
Here are 4 emerging treatments that could improve ADHD management by offering alternatives to stimulants and addressing broader neurochemical pathways 🧵👇

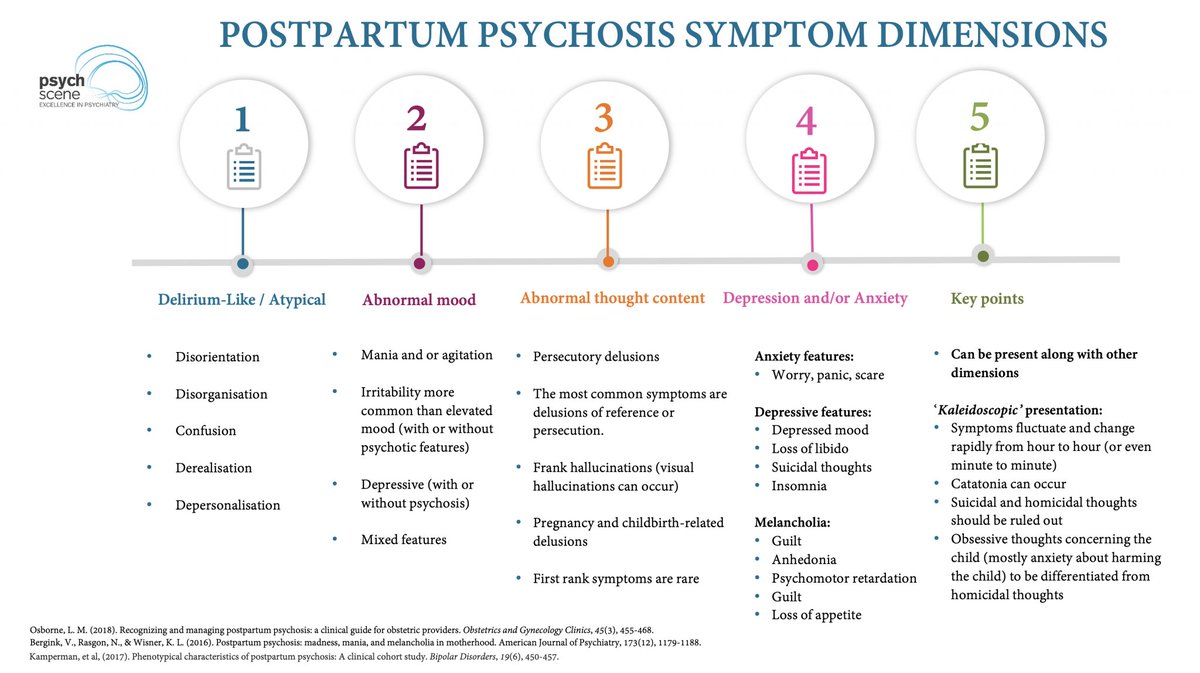
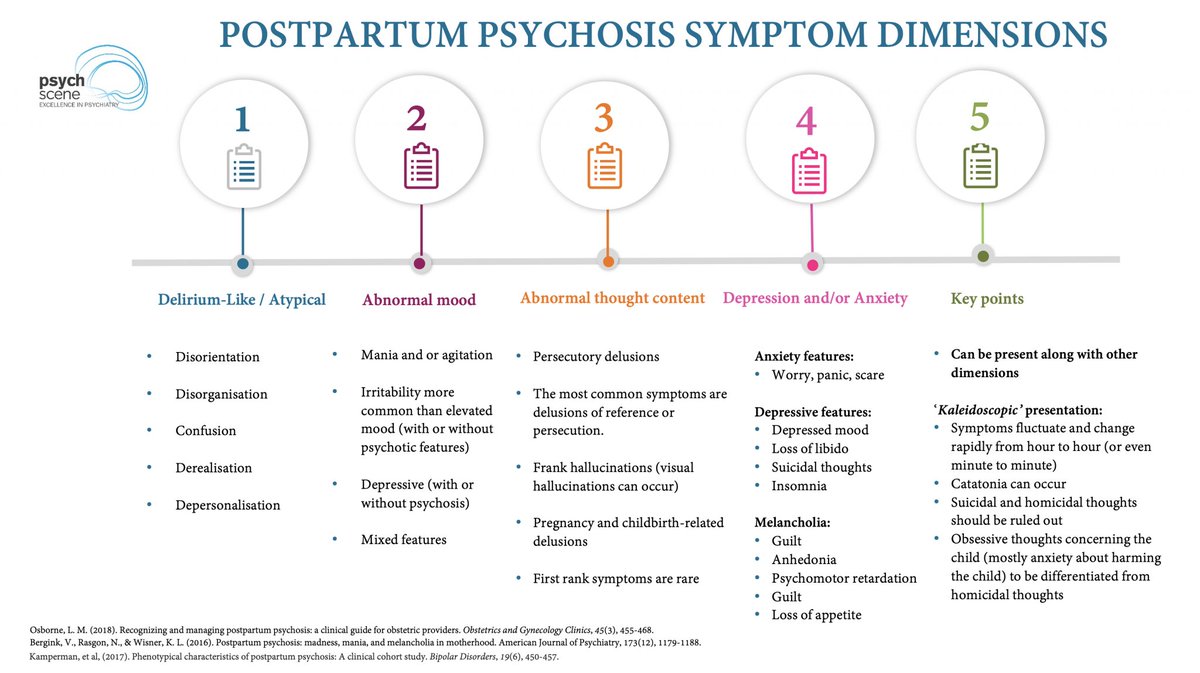
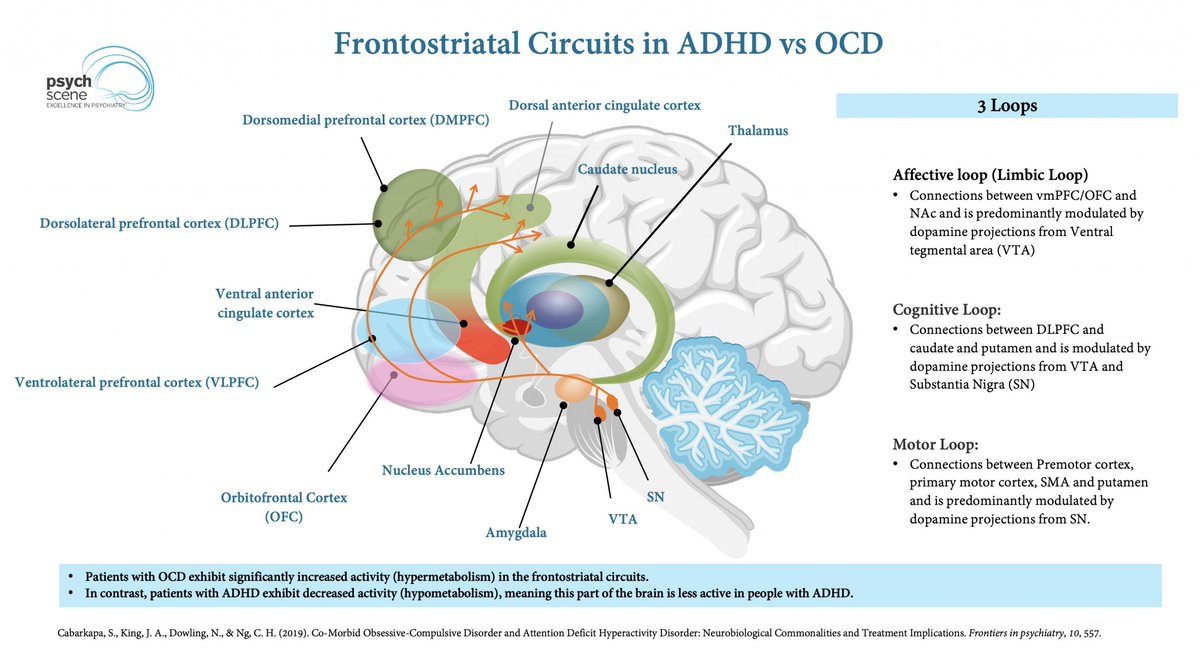
The Cortico-Striatal-Thalamo-Cortical (CSTC) Loop & ADHD
ADHD is linked to dopaminergic & noradrenergic dysfunction in CSTC circuits, affecting:
- Attention
- Impulse control
- Executive function
Targeting these circuits is key to optimising treatment strategies.
ADHD is linked to dopaminergic & noradrenergic dysfunction in CSTC circuits, affecting:
- Attention
- Impulse control
- Executive function
Targeting these circuits is key to optimising treatment strategies.
1. Viloxazine (Qelbree) – A serotonin-norepinephrine modulating agent (SNMA)
- Increases serotonin in the prefrontal cortex
- Modulates norepinephrine & dopamine
- FDA-approved (2021) for ages 6-17
📌 Effective for inattention & impulsivity but carries a black box warning for suicidality.
💡 Psych Scene Tip: Consider Viloxazine for stimulant non-responders, but monitor closely for mood changes.
(Source: Yu et al., 2020)
- Increases serotonin in the prefrontal cortex
- Modulates norepinephrine & dopamine
- FDA-approved (2021) for ages 6-17
📌 Effective for inattention & impulsivity but carries a black box warning for suicidality.
💡 Psych Scene Tip: Consider Viloxazine for stimulant non-responders, but monitor closely for mood changes.
(Source: Yu et al., 2020)
2. Azstarys (Serdexmethylphenidate + Dexmethylphenidate) – A next-gen stimulant
- 70% prodrug (SDX), 30% active dexmethylphenidate
- Longer duration than standard methylphenidate
- FDA-approved (2021) for ages 6+
📌 Designed for once-daily dosing with smoother onset & offset.
💡 Psych Scene Tip: Azstarys provides stimulant benefits with reduced rebound effects—consider for patients needing smoother coverage.
(Source: AZSTARYS Prescribing Information, 2021)
- 70% prodrug (SDX), 30% active dexmethylphenidate
- Longer duration than standard methylphenidate
- FDA-approved (2021) for ages 6+
📌 Designed for once-daily dosing with smoother onset & offset.
💡 Psych Scene Tip: Azstarys provides stimulant benefits with reduced rebound effects—consider for patients needing smoother coverage.
(Source: AZSTARYS Prescribing Information, 2021)
3. Mazindol – A noradrenaline & dopamine reuptake inhibitor (NDRI)
- Originally developed for obesity, now repurposed for ADHD
- Modulates serotonin, orexin, & histamine
- Lower abuse potential than stimulants
📌 Promising for adults & treatment-resistant ADHD.
💡 Psych Scene Tip: Mazindol’s lower abuse risk makes it a potential option for ADHD patients with a history of substance use disorder.
(Source: Wigal et al., 2018)
- Originally developed for obesity, now repurposed for ADHD
- Modulates serotonin, orexin, & histamine
- Lower abuse potential than stimulants
📌 Promising for adults & treatment-resistant ADHD.
💡 Psych Scene Tip: Mazindol’s lower abuse risk makes it a potential option for ADHD patients with a history of substance use disorder.
(Source: Wigal et al., 2018)
4. Centanafadine – A triple reuptake inhibitor (noradrenaline > dopamine > serotonin)
- Increases noradrenaline & dopamine by 300-400%
- Significant symptom reduction by week 1
- Still in clinical trials, but promising for adult ADHD
📌 A potential non-stimulant alternative to current treatments.
💡 Psych Scene Tip: Centanafadine’s rapid onset could benefit patients who need faster symptom relief than standard non-stimulants.
(Source: Wigal et al., 2020)
- Increases noradrenaline & dopamine by 300-400%
- Significant symptom reduction by week 1
- Still in clinical trials, but promising for adult ADHD
📌 A potential non-stimulant alternative to current treatments.
💡 Psych Scene Tip: Centanafadine’s rapid onset could benefit patients who need faster symptom relief than standard non-stimulants.
(Source: Wigal et al., 2020)
What This Means for ADHD Treatment
✅ Broader mechanisms beyond dopamine
✅ Better tolerability vs. traditional stimulants
✅ Options for stimulant non-responders
Want to go deeper into ADHD psychopharmacology?
✅ Broader mechanisms beyond dopamine
✅ Better tolerability vs. traditional stimulants
✅ Options for stimulant non-responders
Want to go deeper into ADHD psychopharmacology?
Explore the neurobiology of ADHD, stimulant vs. non-stimulant, & prescribing considerations in our expert-led course, “Neuroscience and Advanced Psychopharmacology of ADHD – A Comprehensive Guide” on The Academy.
psychscene.co/4gTqTjq
psychscene.co/4gTqTjq
• • •
Missing some Tweet in this thread? You can try to
force a refresh