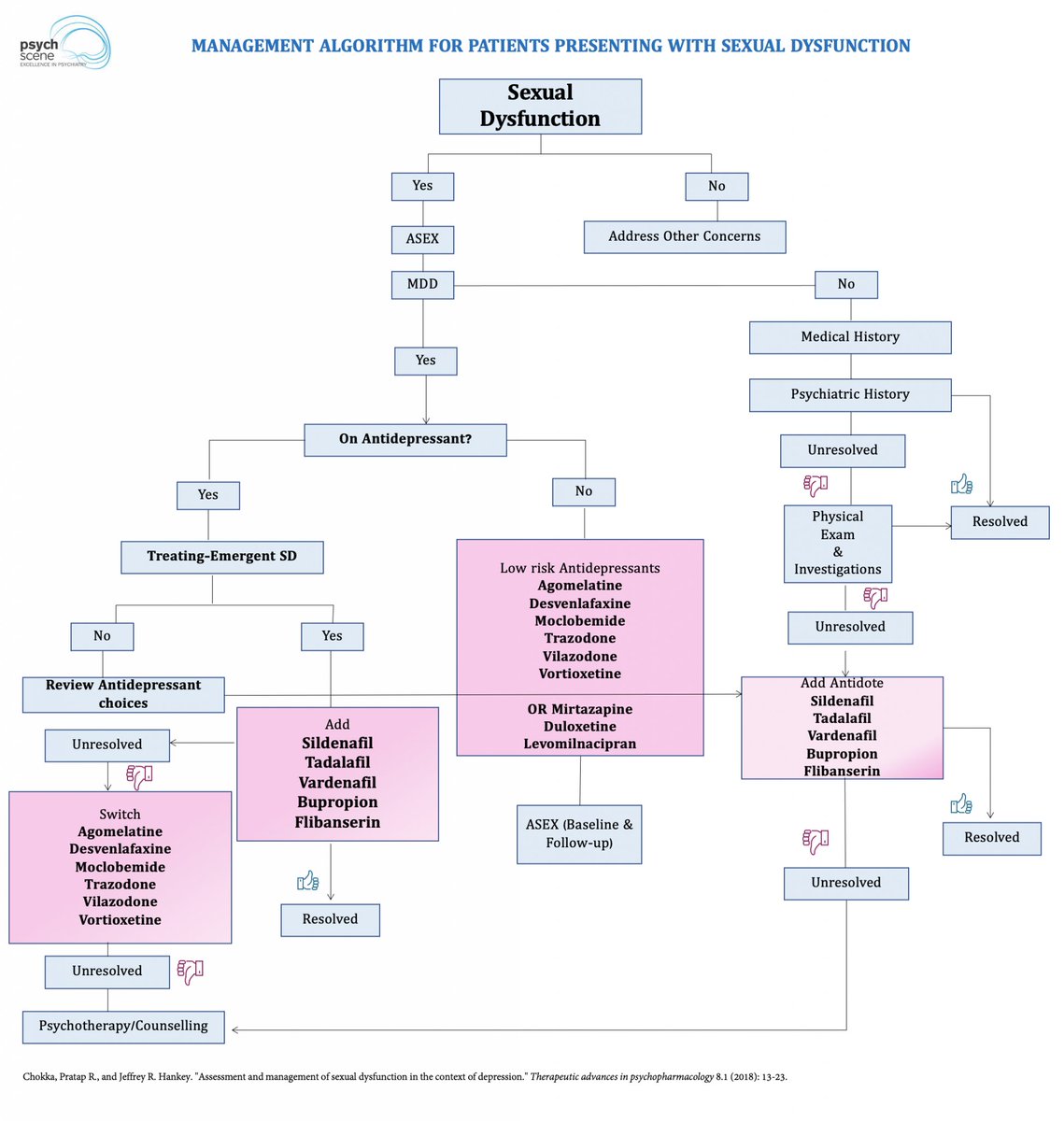
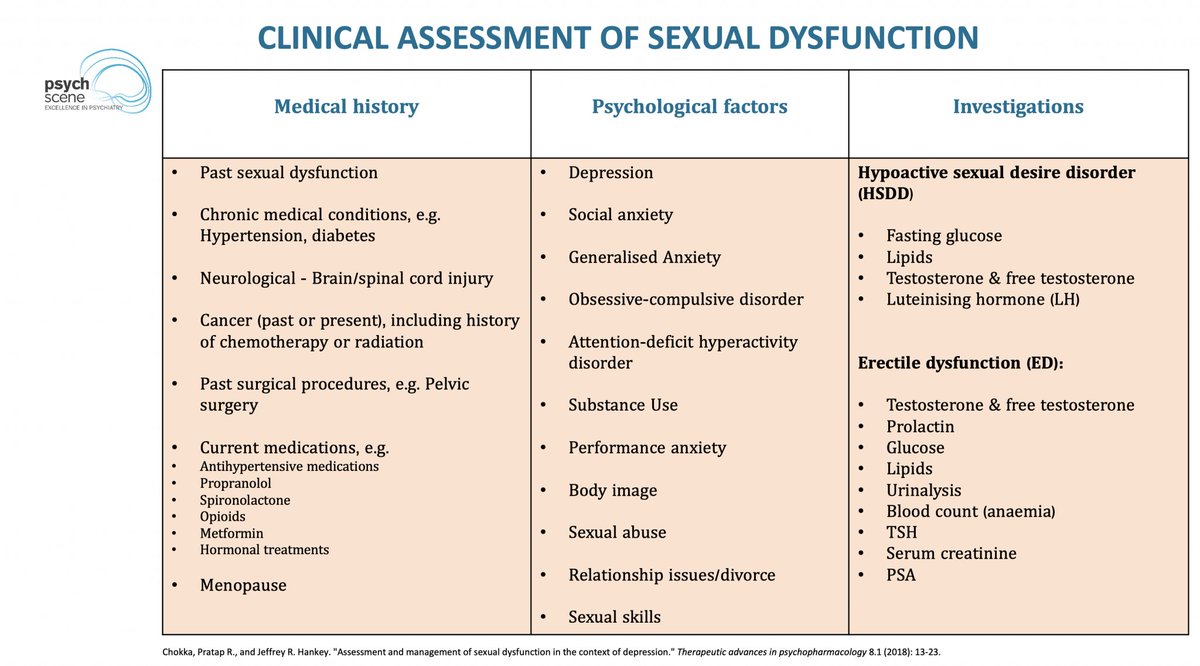
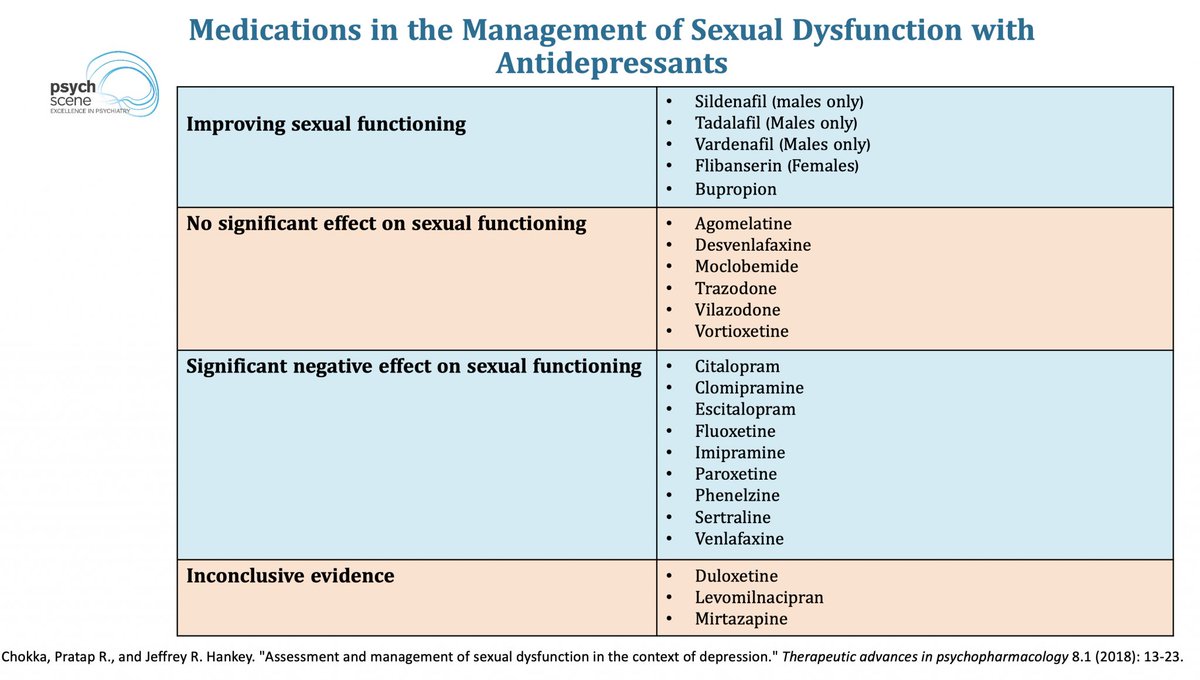
At birth, the brain is a blank slate, but not an empty one.
A newborn's survival depends on basic needs—warmth, nourishment, and safety.
But how does this evolve into complex emotions, consciousness, and psychiatric disorders?
Let's explore how core emotional circuits shape mental health. 👇🧵
A newborn's survival depends on basic needs—warmth, nourishment, and safety.
But how does this evolve into complex emotions, consciousness, and psychiatric disorders?
Let's explore how core emotional circuits shape mental health. 👇🧵

The Neuroscience of Emotions & Mental Health
Before we feel emotions, we experience affect—raw physiological states.
These early signals shape how we perceive the world before cognition develops.
🔹 Hunger → distress
🔹 Warmth → calm
🔹 Separation → panic
These primal circuits lay the foundation for future emotional responses.
Before we feel emotions, we experience affect—raw physiological states.
These early signals shape how we perceive the world before cognition develops.
🔹 Hunger → distress
🔹 Warmth → calm
🔹 Separation → panic
These primal circuits lay the foundation for future emotional responses.
Three Levels of Affects (Panksepp):
🧩 Homeostatic (Drives) – Survival instincts (hunger, thirst).
🧠 Emotional (Instincts) – Innate reactions (fear, attachment).
👁️ Sensory (Reflexes) – Pain, pleasure, and sensory processing.
These systems interact to form the basis of emotions and psychiatric disorders.
🧩 Homeostatic (Drives) – Survival instincts (hunger, thirst).
🧠 Emotional (Instincts) – Innate reactions (fear, attachment).
👁️ Sensory (Reflexes) – Pain, pleasure, and sensory processing.
These systems interact to form the basis of emotions and psychiatric disorders.

How Emotions Take Shape
Early experiences sculpt emotional circuits that determine resilience or vulnerability.
🔹 Secure attachment fosters emotional regulation.
🔹 Trauma or neglect disrupts these circuits, increasing psychiatric risk.
💡 Psych Scene Tip: Early interventions improve emotional resilience.
Early experiences sculpt emotional circuits that determine resilience or vulnerability.
🔹 Secure attachment fosters emotional regulation.
🔹 Trauma or neglect disrupts these circuits, increasing psychiatric risk.
💡 Psych Scene Tip: Early interventions improve emotional resilience.
The Seven Core Emotional Systems (Panksepp)
🔎 SEEKING – Motivation & reward
😨 FEAR – Threat response
😡 RAGE – Defensive aggression
💖 CARE – Nurturing & bonding
🔥 LUST – Reproductive drive
😭 PANIC/GRIEF – Attachment & loss
😆 PLAY – Social learning
Each plays a role in mental health, emotional processing, and psychiatric conditions.
🔎 SEEKING – Motivation & reward
😨 FEAR – Threat response
😡 RAGE – Defensive aggression
💖 CARE – Nurturing & bonding
🔥 LUST – Reproductive drive
😭 PANIC/GRIEF – Attachment & loss
😆 PLAY – Social learning
Each plays a role in mental health, emotional processing, and psychiatric conditions.
Depression: When the SEEKING System Shuts Down
Depression isn’t just sadness. It’s a failure of motivation and reward processing.
- Low dopamine reduces goal-directed behaviour.
- Excess PANIC/GRIEF leads to social withdrawal.
- Neuroinflammation worsens anhedonia.
💡 Psych Scene Tip: Behavioural activation restores motivation and reward.
Depression isn’t just sadness. It’s a failure of motivation and reward processing.
- Low dopamine reduces goal-directed behaviour.
- Excess PANIC/GRIEF leads to social withdrawal.
- Neuroinflammation worsens anhedonia.
💡 Psych Scene Tip: Behavioural activation restores motivation and reward.
Anxiety: The Brain Stuck in FEAR Mode
Anxiety isn’t just overthinking. It’s a hyperactive threat system.
- Overactive amygdala = heightened fear response.
- Dysregulated stress hormones = chronic tension.
- Weak prefrontal control = poor emotional regulation.
💡 Psych Scene Tip: Exposure therapy helps rewire fear pathways.
Anxiety isn’t just overthinking. It’s a hyperactive threat system.
- Overactive amygdala = heightened fear response.
- Dysregulated stress hormones = chronic tension.
- Weak prefrontal control = poor emotional regulation.
💡 Psych Scene Tip: Exposure therapy helps rewire fear pathways.

PTSD presents in two neurobiological patterns:
⚡ Hyperarousal → High fear response (fight/flight/freeze).
😶 Dissociation → Emotional shutdown due to excessive inhibition.
Treatment must match the subtype for effective intervention.
⚡ Hyperarousal → High fear response (fight/flight/freeze).
😶 Dissociation → Emotional shutdown due to excessive inhibition.
Treatment must match the subtype for effective intervention.
Addiction: The SEEKING System Hijacked
Substances artificially spike dopamine, creating false reward signals.
- Over time, natural rewards lose their appeal.
- The PANIC/GRIEF system fuels withdrawal.
- Impulse control weakens, reinforcing the cycle.
💡 Psych Scene Tip: Recovery focuses on restoring natural SEEKING behaviours.
Substances artificially spike dopamine, creating false reward signals.
- Over time, natural rewards lose their appeal.
- The PANIC/GRIEF system fuels withdrawal.
- Impulse control weakens, reinforcing the cycle.
💡 Psych Scene Tip: Recovery focuses on restoring natural SEEKING behaviours.

The Brain Can Rewire
Neuroplasticity allows emotional circuits to adapt with:
✅ Psychotherapy (CBT, EMDR, trauma-focused approaches)
✅ Pharmacotherapy (SSRIs, dopamine modulators)
✅ Lifestyle changes (exercise, mindfulness, social connection)
Mental illness is not a fixed state. The brain can heal.
Neuroplasticity allows emotional circuits to adapt with:
✅ Psychotherapy (CBT, EMDR, trauma-focused approaches)
✅ Pharmacotherapy (SSRIs, dopamine modulators)
✅ Lifestyle changes (exercise, mindfulness, social connection)
Mental illness is not a fixed state. The brain can heal.
What Clinicians Need to Know
- Psychiatric disorders reflect emotional circuit dysregulation.
- Depression = SEEKING dysfunction.
- Anxiety = FEAR dysregulation.
- Addiction = SEEKING hijacked by artificial rewards.
Targeting the right emotional circuits is key to effective treatment.
- Psychiatric disorders reflect emotional circuit dysregulation.
- Depression = SEEKING dysfunction.
- Anxiety = FEAR dysregulation.
- Addiction = SEEKING hijacked by artificial rewards.
Targeting the right emotional circuits is key to effective treatment.
Want to dive deeper into the neuroscience of emotions?
Read the full article, "The Neuroscience of Emotions: Clinical Relevance for Understanding Depression, Anxiety, and Addiction," by Dr Sanil Rege.
Explore how core affective systems shape mental illness and discover evidence-based treatment strategies here:
psychscene.co/4bgG9Ga
Read the full article, "The Neuroscience of Emotions: Clinical Relevance for Understanding Depression, Anxiety, and Addiction," by Dr Sanil Rege.
Explore how core affective systems shape mental illness and discover evidence-based treatment strategies here:
psychscene.co/4bgG9Ga
• • •
Missing some Tweet in this thread? You can try to
force a refresh