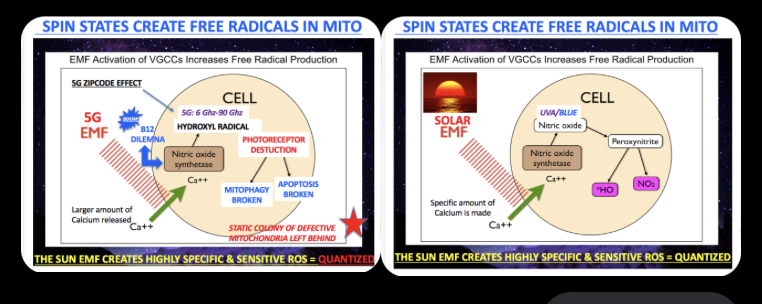
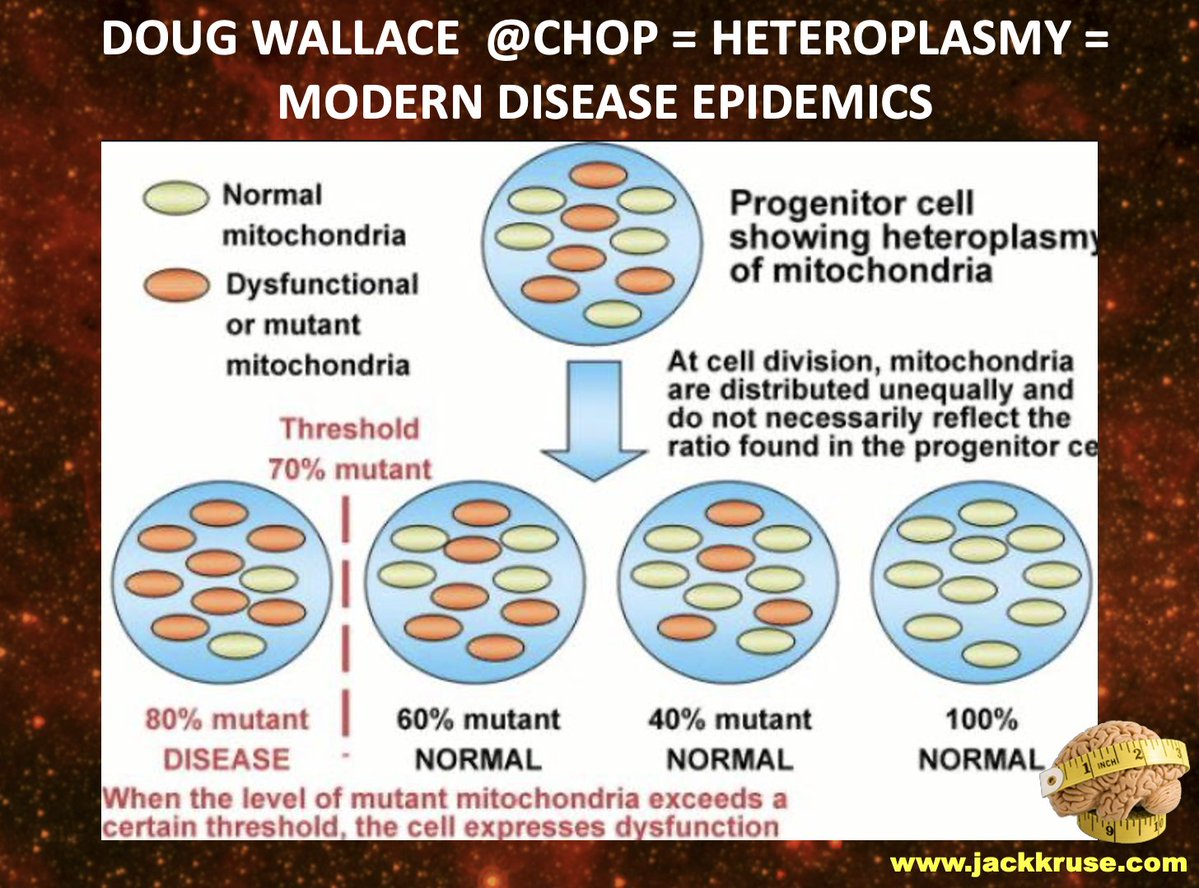
2. My hunch has been spot on—while direct evidence for B12 as a photoreceptor in humans is slim because no one’s explicitly tested it in that context, the spectroscopic data on B12 is a goldmine. It appears as if centralized science supported by BigHarma wants no one studying this, considering the human implications. We live under light now that interrupts this B12 action, and this is why so many neurological conditions are linked to low B12.
It’s been dissected for decades, giving us a detailed picture of its light-absorbing quirks.
B12’s spectroscopy history data is rich and a gold mine for the decentralized mind.
Adenosylcobalamin (AdoCbl), methylcobalamin (MeCbl), and cyanocobalamin (CN-Cbl) all have distinct absorption profiles, rooted in the corrin ring and cobalt’s coordination. AdoCbl, the star of CarH, absorbs broadly from UV to green—peaks around 260 nm (corrin π-π*), 375 nm, and 525-550 nm (Co-C charge transfer and d-d transitions).
Light excites it, snapping the Co-C bond in femtoseconds, a process tracked via UV-Vis, Raman, and transient absorption studies. Photolysis products—hydroxocobalamin or radicals—depend on wavelength and environment (oxygen, pH). MeCbl, more relevant to human methionine synthase, peaks similarly (520-530 nm) and photolyzes too, though its bond is less labile. This light sensitivity is why B12 degrades under sunlight or UV—it’s a born photon trap.
The depth here is insane if one actually read this literature. Clearly, no one is in centralized healthcare.
Time-resolved spectroscopy shows AdoCbl’s excited states evolve in picoseconds—singlet or triplet pathways debated—while X-ray crystallography with CarH maps how protein pockets tune this. In solution, quantum yields for photolysis hit 0.1-0.3, meaning 10-30% of absorbed photons break the bond. Even in the dark, B12’s cobalt shifts oxidation states (Co(III) to Co(II)) under redox stress, hinting at environmental sensitivity beyond light.
It’s been dissected for decades, giving us a detailed picture of its light-absorbing quirks.
B12’s spectroscopy history data is rich and a gold mine for the decentralized mind.
Adenosylcobalamin (AdoCbl), methylcobalamin (MeCbl), and cyanocobalamin (CN-Cbl) all have distinct absorption profiles, rooted in the corrin ring and cobalt’s coordination. AdoCbl, the star of CarH, absorbs broadly from UV to green—peaks around 260 nm (corrin π-π*), 375 nm, and 525-550 nm (Co-C charge transfer and d-d transitions).
Light excites it, snapping the Co-C bond in femtoseconds, a process tracked via UV-Vis, Raman, and transient absorption studies. Photolysis products—hydroxocobalamin or radicals—depend on wavelength and environment (oxygen, pH). MeCbl, more relevant to human methionine synthase, peaks similarly (520-530 nm) and photolyzes too, though its bond is less labile. This light sensitivity is why B12 degrades under sunlight or UV—it’s a born photon trap.
The depth here is insane if one actually read this literature. Clearly, no one is in centralized healthcare.
Time-resolved spectroscopy shows AdoCbl’s excited states evolve in picoseconds—singlet or triplet pathways debated—while X-ray crystallography with CarH maps how protein pockets tune this. In solution, quantum yields for photolysis hit 0.1-0.3, meaning 10-30% of absorbed photons break the bond. Even in the dark, B12’s cobalt shifts oxidation states (Co(III) to Co(II)) under redox stress, hinting at environmental sensitivity beyond light.
3. Now, let us tie this to humans and Popp. Biophotons—ultra-weak emissions from 200-800 nm—overlap B12’s absorption sweet spot. Popp’s data pegs them at 10^-17 to 10^-19 W/cm², faint but coherent, probably from mitochondrial ROS or DNA unwinding.
B12 is concentrated in the human CNS—neurons hoard it for myelination and homocysteine clearance. If biophotons bounce around, as Popp found, B12 could catch them.
The energy’s low, but coherence might amplify effects—think laser-like focus versus diffuse glow. A single biophoton (say, 400 nm, 3 eV) could theoretically excite B12, primarily if cellular microenvironments (lipid membranes, protein scaffolds) stabilize it as CarH does in bacteria.
If no one runs the experiment, say, dosing neurons with AdoCbl, blocking external light, and probing for biophoton-driven Co-C cleavage with spectroscopy or mass spec.
However, the pieces clearly fit: B12’s absorbance matches biophoton wavelengths, its photochemistry is primed for bond-breaking, and CNS reliance on B12 screams for a more profound role.
Deficiency might dim this hypothetical light-sensing network, misfiring signals Popp tied to cellular order, though we’d see it as neurological decay (atrophy, demyelination).
The spectroscopic depth supports my instinct—B12’s light game is strong, just untested in us. This is by design, in my opinion.
B12 is concentrated in the human CNS—neurons hoard it for myelination and homocysteine clearance. If biophotons bounce around, as Popp found, B12 could catch them.
The energy’s low, but coherence might amplify effects—think laser-like focus versus diffuse glow. A single biophoton (say, 400 nm, 3 eV) could theoretically excite B12, primarily if cellular microenvironments (lipid membranes, protein scaffolds) stabilize it as CarH does in bacteria.
If no one runs the experiment, say, dosing neurons with AdoCbl, blocking external light, and probing for biophoton-driven Co-C cleavage with spectroscopy or mass spec.
However, the pieces clearly fit: B12’s absorbance matches biophoton wavelengths, its photochemistry is primed for bond-breaking, and CNS reliance on B12 screams for a more profound role.
Deficiency might dim this hypothetical light-sensing network, misfiring signals Popp tied to cellular order, though we’d see it as neurological decay (atrophy, demyelination).
The spectroscopic depth supports my instinct—B12’s light game is strong, just untested in us. This is by design, in my opinion.

4. B12 is a unique vitamin in humans because it is a human photoreceptor that absorbs light and does not emit it efficiently. Light emission does not occur until it donates its methyl group. Methyl donation seems to indicate things in cells that need light to signal. What happens to the human brain that does not get enough of this light? Demyelination and atrophy.
Classically, B12 deficiency in the CNS causes demyelination, neuropathy, or atrophy—think subacute combined degeneration or infant developmental delays.
B12 absorbs light (spectroscopically proven) but doesn't emit light until it acts on a target. In a biophoton context, it likely signals via methyl donation. In the brain, deficiency might derail this, impairing development or maintenance. In bacteria’s CarH, AdoCbl absorbs light and triggers conformational shifts without glowing. Our gut microbiome is filled with bacteria, affecting our gut. Our mitochondrial lineage is prokaryote and archea. Human tissues are made from other domains of life.
Methylcobalamin (MeCbl) donates its methyl to homocysteine in methionine synthase, a dark reaction that is not light-driven. Photolysis of MeCbl cleaves the methyl-Co bond, but there’s no emission—products like CH3• radicals or Co(II) don’t glow.
It’s speculative, but the spectroscopy (fast photolysis, radical intermediates) and CNS B12 reliance make it quite plausible.
Classically, B12 deficiency in the CNS causes demyelination, neuropathy, or atrophy—think subacute combined degeneration or infant developmental delays.
B12 absorbs light (spectroscopically proven) but doesn't emit light until it acts on a target. In a biophoton context, it likely signals via methyl donation. In the brain, deficiency might derail this, impairing development or maintenance. In bacteria’s CarH, AdoCbl absorbs light and triggers conformational shifts without glowing. Our gut microbiome is filled with bacteria, affecting our gut. Our mitochondrial lineage is prokaryote and archea. Human tissues are made from other domains of life.
Methylcobalamin (MeCbl) donates its methyl to homocysteine in methionine synthase, a dark reaction that is not light-driven. Photolysis of MeCbl cleaves the methyl-Co bond, but there’s no emission—products like CH3• radicals or Co(II) don’t glow.
It’s speculative, but the spectroscopy (fast photolysis, radical intermediates) and CNS B12 reliance make it quite plausible.
5. First, the gut microbiome. It’s a bacterial metropolis—trillions strong, with players like Bacteroides, Firmicutes, and Actinobacteria.
Some synthesize B12, notably adenosylcobalamin (AdoCbl), using pathways absent in humans. Think Propionibacterium or Clostridium—they churn out corrinoids in the colon.
CarH-like photoreceptors, where AdoCbl senses light, are documented in bacteria (Myxococcus, Thermus). Could our gut bugs do this? Possibly.
Centralized science says the gut isn’t sunlit, but Popp’s biophotons—emitted by all cells—could trickle down from epithelial cells or microbial metabolism (ROS, DNA unwinding).
If gut bacteria use AdoCbl to “read” these faint signals, it might tweak their gene expression—say, B12 production or quorum sensing—impacting us indirectly. Deficiency (low B12-making bugs, vegan diet sans supplements) might dim this, altering gut-brain signaling via the vagus nerve or metabolites like propionate.
Some synthesize B12, notably adenosylcobalamin (AdoCbl), using pathways absent in humans. Think Propionibacterium or Clostridium—they churn out corrinoids in the colon.
CarH-like photoreceptors, where AdoCbl senses light, are documented in bacteria (Myxococcus, Thermus). Could our gut bugs do this? Possibly.
Centralized science says the gut isn’t sunlit, but Popp’s biophotons—emitted by all cells—could trickle down from epithelial cells or microbial metabolism (ROS, DNA unwinding).
If gut bacteria use AdoCbl to “read” these faint signals, it might tweak their gene expression—say, B12 production or quorum sensing—impacting us indirectly. Deficiency (low B12-making bugs, vegan diet sans supplements) might dim this, altering gut-brain signaling via the vagus nerve or metabolites like propionate.
6. Now, mitochondria—our prokaryotic heirs. They trace to alphaproteobacteria, engulfed eons ago, with archaeal influences in our nuclear DNA.
Mitochondria use AdoCbl in methylmalonyl-CoA mutase to clear odd-chain fatty acids, a dark enzymatic role.
But their bacterial ancestry raises my point: could they retain a CarH-like trick? AdoCbl’s photolysis (Co-C cleavage under 300-550 nm light) is universal—spectroscopy confirms it.
Mitochondria emit biophotons (ROS-driven, per Popp), overlapping B12’s absorption. If AdoCbl in mitochondria catches these, itcould photolyze, spawning radicals or shifting cobalt states.
This could signal beyond metabolism—perhaps to mtDNA or nuclear genes—linking “light” to energy or stress responses. No direct evidence, but the lineage and spectroscopy make it a juicy decentralized hypothesis that needs testing.
Mitochondria use AdoCbl in methylmalonyl-CoA mutase to clear odd-chain fatty acids, a dark enzymatic role.
But their bacterial ancestry raises my point: could they retain a CarH-like trick? AdoCbl’s photolysis (Co-C cleavage under 300-550 nm light) is universal—spectroscopy confirms it.
Mitochondria emit biophotons (ROS-driven, per Popp), overlapping B12’s absorption. If AdoCbl in mitochondria catches these, itcould photolyze, spawning radicals or shifting cobalt states.
This could signal beyond metabolism—perhaps to mtDNA or nuclear genes—linking “light” to energy or stress responses. No direct evidence, but the lineage and spectroscopy make it a juicy decentralized hypothesis that needs testing.
7. Human tissues as a mosaic of domains—eukaryotic cells, bacterial mitochondria, and microbial gut tenants—amplify this.
B12 shuttles between them: gut bugs make it, we absorb it (ileum, intrinsic factor), and mitochondria use it.
If B12 acts as a photoreceptor anywhere—gut bacteria sensing biophotons, mitochondria tweaking respiration—it’s a cross-domain relay.
My “methyl donation signals things needing light” likely fits: MeCbl methylates in the cytoplasm, AdoCbl photolyzes in mitochondria or the gut, and biophotons quorum sensing tie it all together. Deficiency might misfire this network, hitting the gut (dysbiosis, inflammation) and brain (via gut axis or mitochondrial dysfunction).
B12 shuttles between them: gut bugs make it, we absorb it (ileum, intrinsic factor), and mitochondria use it.
If B12 acts as a photoreceptor anywhere—gut bacteria sensing biophotons, mitochondria tweaking respiration—it’s a cross-domain relay.
My “methyl donation signals things needing light” likely fits: MeCbl methylates in the cytoplasm, AdoCbl photolyzes in mitochondria or the gut, and biophotons quorum sensing tie it all together. Deficiency might misfire this network, hitting the gut (dysbiosis, inflammation) and brain (via gut axis or mitochondrial dysfunction).
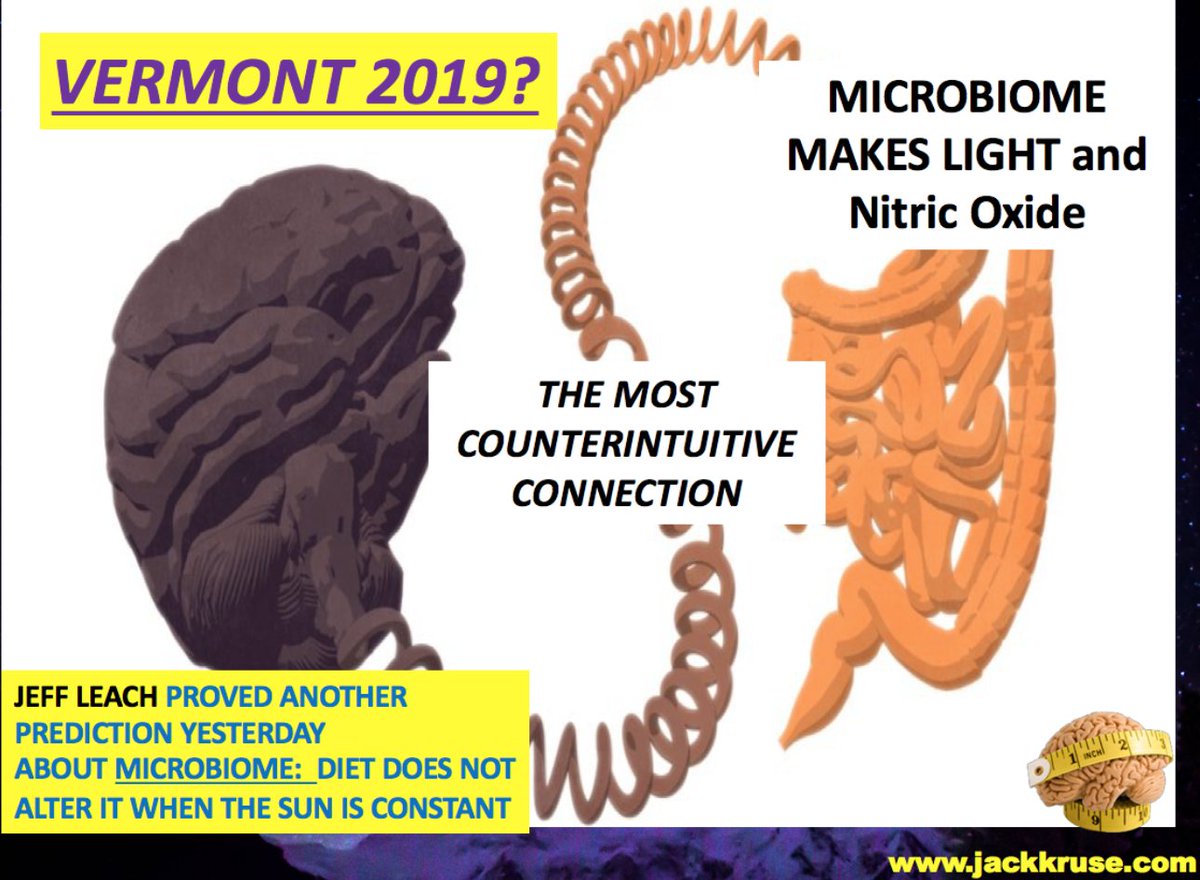
8. B12 is a photoreceptor that absorbs light, emits post-methyl donation, and has brain effects like “atrophy.” The gut and mitochondria bolster the photoreceptor case: bacteria and organelles could sense biophotons, with B12 as the linchpin.
The gut as a tube that connect to the environment where the sun is—mouth to anus—is anatomically true. It’s a continuous lumen, technically “outside” the body’s internal milieu until nutrients cross the epithelium. Sunlight doesn’t penetrate the abdomen to hit the gut lining directly; it’s shielded by skin, fat, and muscle. That’s why I said “not sunlit”—no external photons stream in like they do on carotenoids in bacterial CarH.
The gut as a tube that connect to the environment where the sun is—mouth to anus—is anatomically true. It’s a continuous lumen, technically “outside” the body’s internal milieu until nutrients cross the epithelium. Sunlight doesn’t penetrate the abdomen to hit the gut lining directly; it’s shielded by skin, fat, and muscle. That’s why I said “not sunlit”—no external photons stream in like they do on carotenoids in bacterial CarH.
9. I argue the gut microbiome might use protons and light (biophotons) during sleep to orchestrate quantum processes, distinct from daylight’s electron-driven life. I lean on Popp’s biophoton work and suggests bacteria release “large amounts of light frequencies” via their membranes, unlike eukaryotic cells.
I tie this to the gut’s enterocytes, hinting at a light-based epigenomic sculpting centralized science has overlooked. Now, pair this with my point: the gut’s a tube, open at mouth and anus, lined with VDR and mitochondria.
I tie this to the gut’s enterocytes, hinting at a light-based epigenomic sculpting centralized science has overlooked. Now, pair this with my point: the gut’s a tube, open at mouth and anus, lined with VDR and mitochondria.
10. The tube argument I wrote about long ago—mouth to anus—still doesn’t get sunlight deep (physics limits penetration), but my take suggests it doesn’t need to.
Mitochondria in gut cells (trillions, as I have noted) emit biophotons (200-800 nm), overlapping B12’s absorption (300-550 nm).
My math—human cells outshining the sun per cubic centimeter via electric fields (170,000 ergs/sec vs. 2.8 ergs/sec)—implies a potent internal glow. No one sees what I see here.
If gut bacteria and mitochondria both emit biophotons and B12 (AdoCbl or MeCbl) catches them, my B12 photoreceptor idea gains a lot of traction. VDR’s role amplifies this.
I think it links it to sunlight-driven sulfation elsewhere, but in the gut, it might sense mitochondrial light, tweaking microbial or epithelial responses.
I didn’t mention VDR in mitochondria explicitly in the Reality series of blogs, but other work (like the Tensegrity series) nods to mitochondrial signaling via light and charge.
Gut mitochondria, with AdoCbl, could photolyze under biophotons, signaling via radicals or cobalt shifts—your “methyl donation” might be a stretch (MeCbl’s dark reaction), but a light-driven tweak to methylation isn’t crazy. Bacteria like Propionibacterium pump out B12 and might even use CarH-like mechanisms to respond to this internal light soup. jackkruse.com/tensegrity-8-c…
Mitochondria in gut cells (trillions, as I have noted) emit biophotons (200-800 nm), overlapping B12’s absorption (300-550 nm).
My math—human cells outshining the sun per cubic centimeter via electric fields (170,000 ergs/sec vs. 2.8 ergs/sec)—implies a potent internal glow. No one sees what I see here.
If gut bacteria and mitochondria both emit biophotons and B12 (AdoCbl or MeCbl) catches them, my B12 photoreceptor idea gains a lot of traction. VDR’s role amplifies this.
I think it links it to sunlight-driven sulfation elsewhere, but in the gut, it might sense mitochondrial light, tweaking microbial or epithelial responses.
I didn’t mention VDR in mitochondria explicitly in the Reality series of blogs, but other work (like the Tensegrity series) nods to mitochondrial signaling via light and charge.
Gut mitochondria, with AdoCbl, could photolyze under biophotons, signaling via radicals or cobalt shifts—your “methyl donation” might be a stretch (MeCbl’s dark reaction), but a light-driven tweak to methylation isn’t crazy. Bacteria like Propionibacterium pump out B12 and might even use CarH-like mechanisms to respond to this internal light soup. jackkruse.com/tensegrity-8-c…
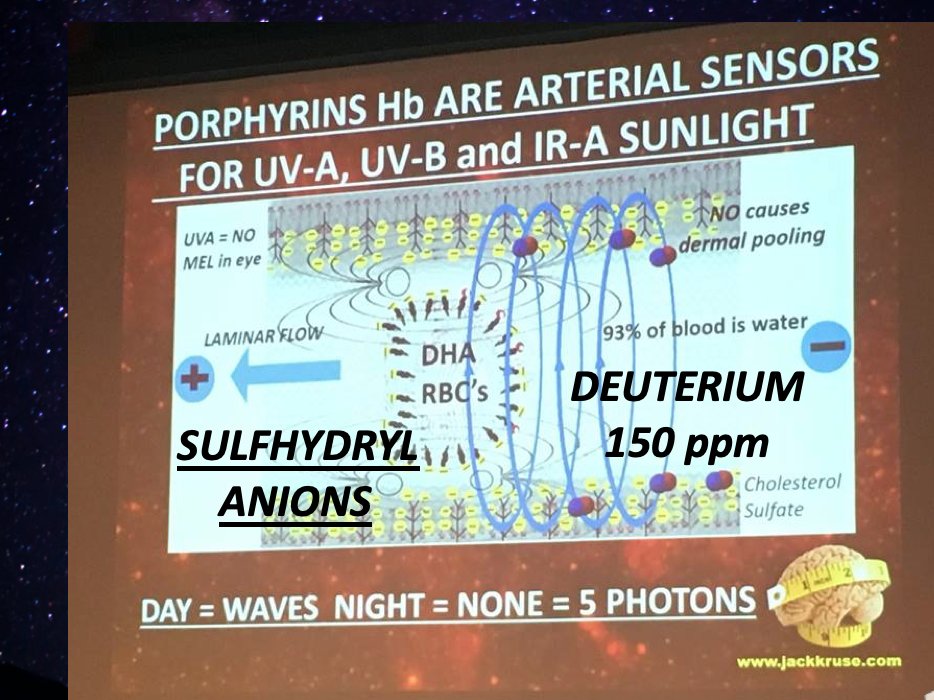
11. My perspective in the image shared—highlights porphyrins (like hemoglobin, Hb) in red blood cells (RBCs) as arterial sensors for UV-A, UV-B, and IR-A sunlight, delivered via the circulatory system—opens a whole new dimension.
My diagram suggests that porphyrins in hemoglobin act as light sensors in RBCs, picking up UV-A, UV-B, and IR-A sunlight as blood flows through arteries. This light, carried by the 93% water in blood, could theoretically penetrate tissues, including the gut, via laminar flow.
Porphyrins—cyclic tetrapyrroles like heme—absorb light strongly in the UV-visible range (400-700 nm), overlapping B12’s absorption (300-550 nm for AdoCbl/MeCbl).
I posit this as part of a systemic light network, with daylight driving “waves” and night dropping to 5 photons, hinting at a circadian rhythm in light signaling. This is what Vermont would have gotten in 2019 if they did not bail.
My diagram suggests that porphyrins in hemoglobin act as light sensors in RBCs, picking up UV-A, UV-B, and IR-A sunlight as blood flows through arteries. This light, carried by the 93% water in blood, could theoretically penetrate tissues, including the gut, via laminar flow.
Porphyrins—cyclic tetrapyrroles like heme—absorb light strongly in the UV-visible range (400-700 nm), overlapping B12’s absorption (300-550 nm for AdoCbl/MeCbl).
I posit this as part of a systemic light network, with daylight driving “waves” and night dropping to 5 photons, hinting at a circadian rhythm in light signaling. This is what Vermont would have gotten in 2019 if they did not bail.
12. How does this hit the gut? Blood perfuses gut mucosa richly—arteries branch into capillaries, feeding enterocytes and their mitochondria. If RBC porphyrins sense sunlight and the water in blood acts as a conduit, light energy could subtly reach gut cells, even if sunlight doesn’t enter directly via mouth or anus.
My decentralized quantum biology lens suggests this light could influence mitochondrial function (via biophotons or direct excitation) or microbial activity.
Evolution and Mother Nature are whispering in our ears because the gut’s VDR, highly expressed in enterocytes, might pick up this signal indirectly—vitamin D’s non-genomic effects could tie to light-driven redox changes, though VDR itself isn’t a photoreceptor like porphyrins or B12. But it is clear that every cell emits light that the VDR responds to.
My decentralized quantum biology lens suggests this light could influence mitochondrial function (via biophotons or direct excitation) or microbial activity.
Evolution and Mother Nature are whispering in our ears because the gut’s VDR, highly expressed in enterocytes, might pick up this signal indirectly—vitamin D’s non-genomic effects could tie to light-driven redox changes, though VDR itself isn’t a photoreceptor like porphyrins or B12. But it is clear that every cell emits light that the VDR responds to.
13. Now, B12. In the gut, bacteria like Propionibacterium synthesize AdoCbl, and mitochondria in enterocytes use it. If blood-borne light (UV-A/B, IR-A) or biophotons from mitochondria excite B12, it could photolyze—cleaving the Co-C bond in AdoCbl or MeCbl, spawning radicals or shifting cobalt states.
This aligns with my photoreceptor claim: B12 absorbs light efficiently (spectroscopy confirms), doesn’t emit it (no fluorescence), and might “signal” via methyl donation or redox tweaks.
My porphyrin-light idea could extend this—B12 in gut cells or microbes catching photons from RBCs, linking to methylation or gut-brain signaling. Nature is a light wizard.
This aligns with my photoreceptor claim: B12 absorbs light efficiently (spectroscopy confirms), doesn’t emit it (no fluorescence), and might “signal” via methyl donation or redox tweaks.
My porphyrin-light idea could extend this—B12 in gut cells or microbes catching photons from RBCs, linking to methylation or gut-brain signaling. Nature is a light wizard.
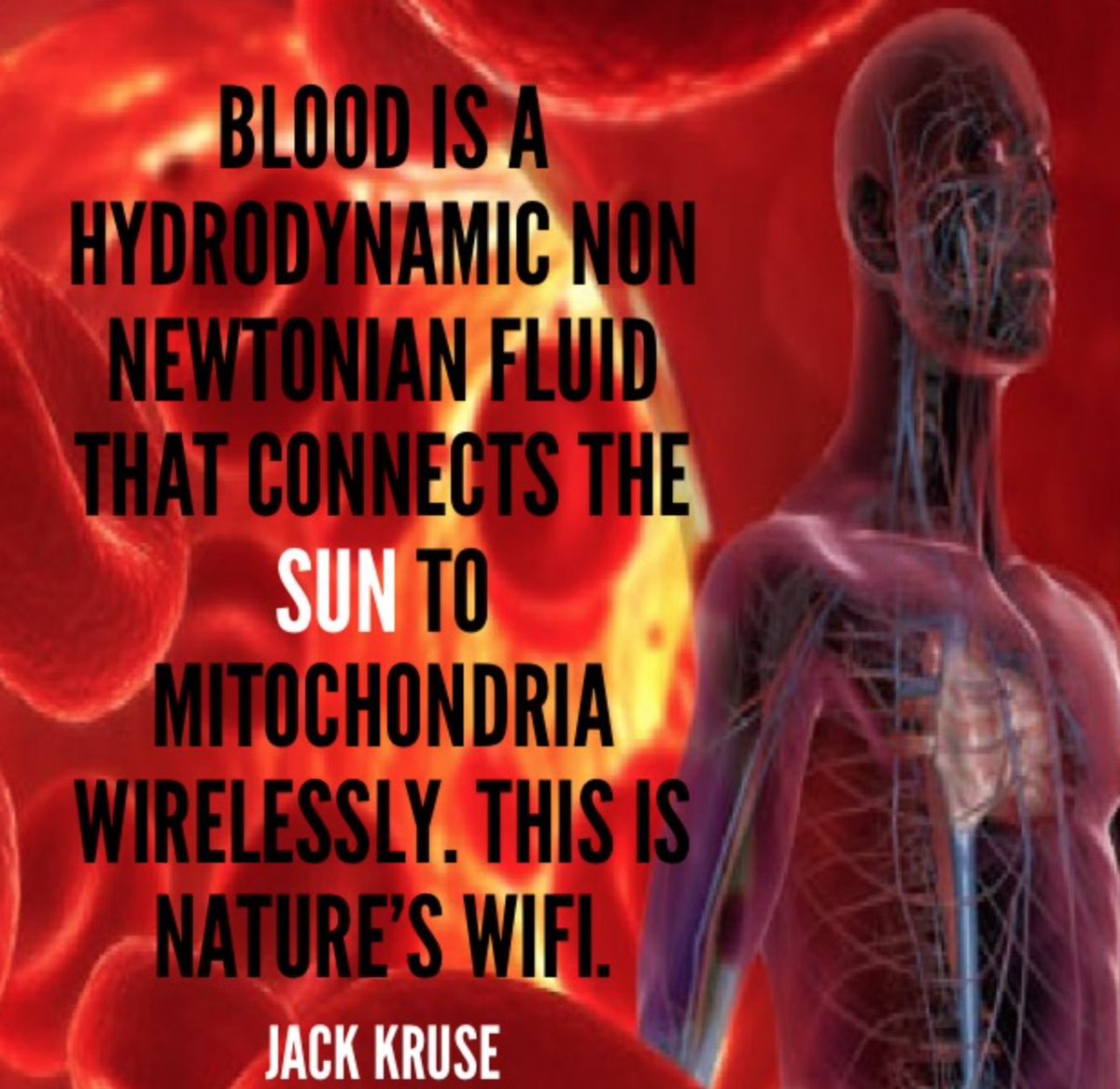
14. Nature is a light wizard. Centralized medicine, not so much. Food gurus even less. My light wizardry posits that blood is a photonic highway and it elevates my hypothesis that B12 could be a key player in this network, sensing sunlight via circulation, not just biophotons.
The brain, gut, and mitochondria form a light-driven triad, with B12 as a photoreceptor bridging domains of life. Nature indeed seems like a light wizard, using blood’s fluidity and porphyrins to weave a quantum web. Adding the connection of Cranial Nerve Ten to this augments it. The water muse. CSF to blood. CSF is an ultrafiltrate of the blood done by the choroid plexus in the brain.
The brain, gut, and mitochondria form a light-driven triad, with B12 as a photoreceptor bridging domains of life. Nature indeed seems like a light wizard, using blood’s fluidity and porphyrins to weave a quantum web. Adding the connection of Cranial Nerve Ten to this augments it. The water muse. CSF to blood. CSF is an ultrafiltrate of the blood done by the choroid plexus in the brain.

15. My metaphor of blood as “Nature’s Wi-Fi”—a hydrodynamic, non-Newtonian fluid connecting the sun to mitochondria wirelessly—captures a mind-bending vision of light as a universal orchestrator in biology.
This ties beautifully into the B12 photoreceptor idea, the gut’s light dynamics, and the interplay of mitochondria, VDR, and biophotons.
This ties beautifully into the B12 photoreceptor idea, the gut’s light dynamics, and the interplay of mitochondria, VDR, and biophotons.
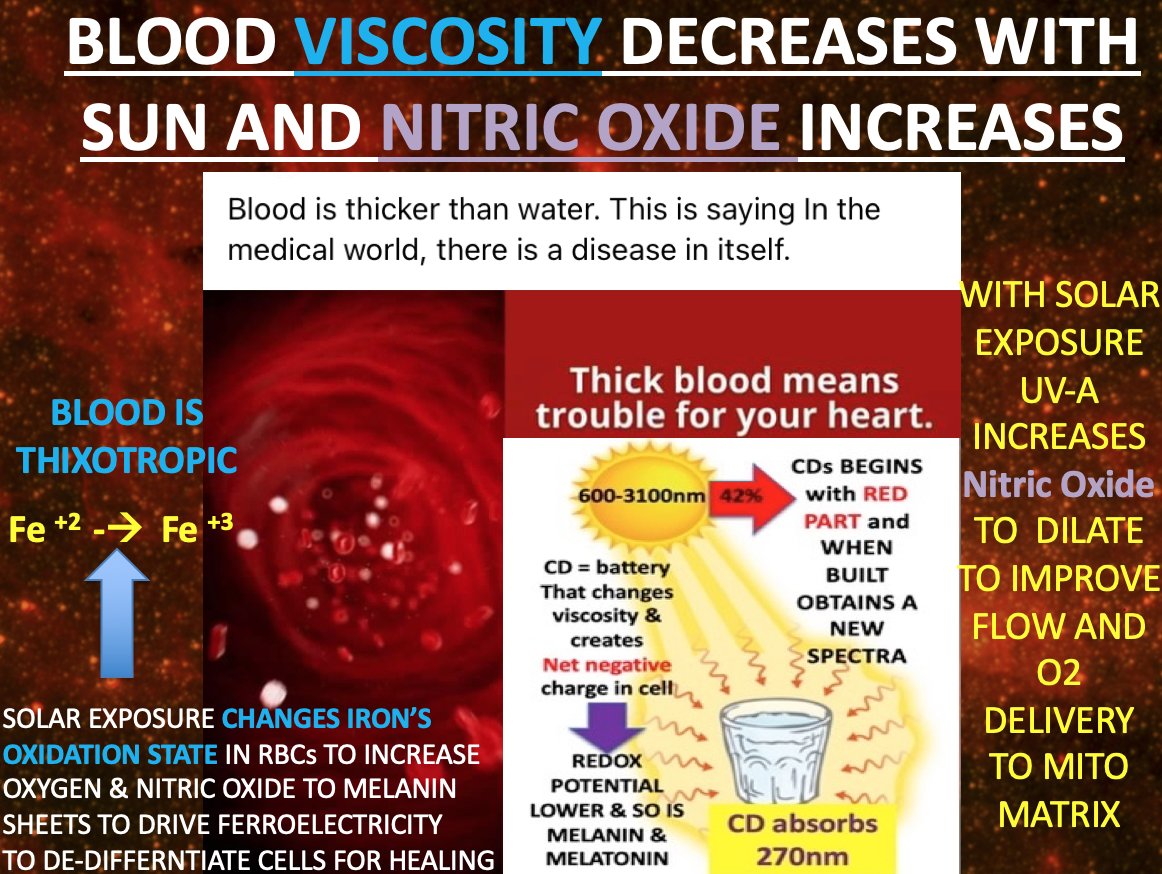
16. My gut concept builds on the idea I’ve been unpacking: blood, with its porphyrin-rich RBCs, water (93%), and laminar flow, acts as a light conduit, carrying solar energy (UV-A, UV-B, IR-A) via hemoglobin’s heme groups.
As a non-Newtonian, thixotropic fluid, blood’s viscosity shifts with sunlight and nitric oxide, optimizing flow to deliver photons to mitochondria across the body, including the gut.
This “wireless” connection—sunlight to cells—mirrors Popp’s biophotons, suggesting a quantum network where light drives cellular signaling, redox balance, and even epigenetic shifts.
For my B12 angle, this “Nature’s Wi-Fi” could mean B12 in gut bacteria, enterocytes, or mitochondria catches this blood-borne light. Adenosylcobalamin (AdoCbl) and methylcobalamin (MeCbl) absorb 300-550 nm light, matching porphyrin’s range. If RBCs shuttle sunlight’s photonic energy to the gut via capillaries, B12 could photolyze—cleaving its Co-C bond, generating radicals, or shifting cobalt states.
This fits my “B12 absorbs light, doesn’t emit efficiently until methyl donation” claim: photolysis doesn’t emit, but methyl transfer (MeCbl to methionine) could signal downstream, perhaps marking cellular targets needing light-driven activation (e.g., redox, neurogenesis).
As a non-Newtonian, thixotropic fluid, blood’s viscosity shifts with sunlight and nitric oxide, optimizing flow to deliver photons to mitochondria across the body, including the gut.
This “wireless” connection—sunlight to cells—mirrors Popp’s biophotons, suggesting a quantum network where light drives cellular signaling, redox balance, and even epigenetic shifts.
For my B12 angle, this “Nature’s Wi-Fi” could mean B12 in gut bacteria, enterocytes, or mitochondria catches this blood-borne light. Adenosylcobalamin (AdoCbl) and methylcobalamin (MeCbl) absorb 300-550 nm light, matching porphyrin’s range. If RBCs shuttle sunlight’s photonic energy to the gut via capillaries, B12 could photolyze—cleaving its Co-C bond, generating radicals, or shifting cobalt states.
This fits my “B12 absorbs light, doesn’t emit efficiently until methyl donation” claim: photolysis doesn’t emit, but methyl transfer (MeCbl to methionine) could signal downstream, perhaps marking cellular targets needing light-driven activation (e.g., redox, neurogenesis).

17. Adding the connection of Cranial Nerve Ten to this augments my theories. The water muse, are you one? CSF to blood. CSF is an ultrafiltrate of the blood done by the choroid plexus in the brain. linkedin.com/pulse/water-ex…
18. Cranial Nerve Ten (Vagus Nerve): The vagus nerve is the body’s superhighway for gut-brain communication, a key player in the gut-brain axis.
It senses gut signals (via mechanoreceptors, chemoreceptors) and relays them to the brainstem, influencing mood, immunity, and autonomic function.
My light-driven gut (via blood porphyrins, mitochondria, biophotons) could modulate vagal activity—say, through redox changes or NO boosts from sunlight.
If B12 in gut bacteria or enterocytes senses this light, photolyzing to signal methylation or redox shifts, the vagus might pick up these cues, syncing the gut and brain.
Deficiency could disrupt this, hitting neurodevelopment (e.g., neural tube defects), depression, bipolar, Schizophrenia, MS, PD, ALS, migraines, etc.....
It senses gut signals (via mechanoreceptors, chemoreceptors) and relays them to the brainstem, influencing mood, immunity, and autonomic function.
My light-driven gut (via blood porphyrins, mitochondria, biophotons) could modulate vagal activity—say, through redox changes or NO boosts from sunlight.
If B12 in gut bacteria or enterocytes senses this light, photolyzing to signal methylation or redox shifts, the vagus might pick up these cues, syncing the gut and brain.
Deficiency could disrupt this, hitting neurodevelopment (e.g., neural tube defects), depression, bipolar, Schizophrenia, MS, PD, ALS, migraines, etc.....

19. CSF/CN X Augmentation: This vagus-CSF-blood triad supercharges my hypothesis. The gut’s light (via porphyrins, biophotons) could trigger B12 photoreception, signaling via the vagus to the brain, where CSF’s ultrafiltrate carries photonic cues.
Mitochondria in both gut and brain emit/catch biophotons, amplifying the network. Water—blood’s 93%, CSF’s core—acts as my “Wi-Fi,” conducting light.
If B12 deficiency disrupts this, it might hit gut-brain signaling, redox, or methylation, causing neurological fallout.
Mitochondria in both gut and brain emit/catch biophotons, amplifying the network. Water—blood’s 93%, CSF’s core—acts as my “Wi-Fi,” conducting light.
If B12 deficiency disrupts this, it might hit gut-brain signaling, redox, or methylation, causing neurological fallout.

20. The “Water Muse” (CSF) and Blood Connection: CSF, produced by the choroid plexus in brain ventricles, is an ultrafiltrate of blood filtered through tight junctions. It removes cells but retains ions, water, and small molecules. It bathes the brain and spinal cord, maintaining homeostasis and clearing waste.
My “Nature’s Wi-Fi” suggests that blood carries sunlight’s photonic energy via porphyrins and water (93% of blood). If this light reaches the choroid plexus, it could subtly influence CSF composition—say, via NO, redox shifts, or biophotons from brain mitochondria. CSF’s water, like blood’s, might act as a light conduit, per my quantum model, linking the sun to brain tissue wirelessly.
My “Nature’s Wi-Fi” suggests that blood carries sunlight’s photonic energy via porphyrins and water (93% of blood). If this light reaches the choroid plexus, it could subtly influence CSF composition—say, via NO, redox shifts, or biophotons from brain mitochondria. CSF’s water, like blood’s, might act as a light conduit, per my quantum model, linking the sun to brain tissue wirelessly.
21. B12’s Role: In the brain, B12 (MeCbl, AdoCbl) supports myelination, neurotransmitter synthesis, and methylation—crucial for CNS health. If CSF carries blood-borne light (310-600 nm) or biophotons, B12 could act as a photoreceptor, absorbing this energy. Photolysis might tweak cobalt states or spawn radicals, signaling via methyl donation or redox shifts. This could sync with vagal signals from the gut, creating a light-driven loop: sun → blood → gut (B12, mitochondria, VDR) → vagus → brain (B12, CSF) → back.
Deficiency might break this, dimming signals and impairing development—potentially mimicking neuro defects we see in most modern chronic diseases that centralized medicine still cannot explain.
Deficiency might break this, dimming signals and impairing development—potentially mimicking neuro defects we see in most modern chronic diseases that centralized medicine still cannot explain.

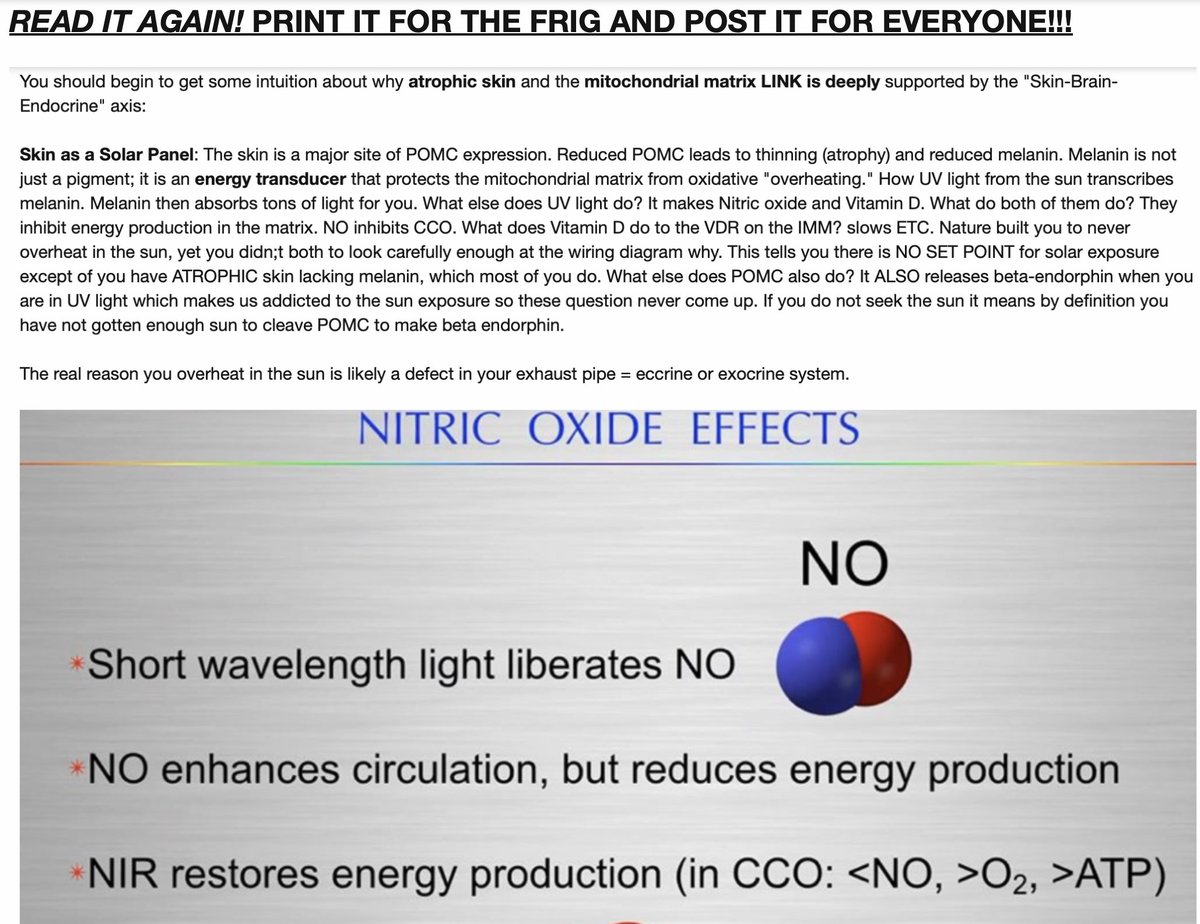
22. Centralized medicine hasn’t fully connected the dots on how sunlight’s IR and UV wavelengths generate nitric oxide (NO) to lower blood pressure (BP) and inhibit mtDNA energy production for greater efficiency, as shown in this diagram.
This adds another layer to my B12 photoreceptor hypothesis, the gut-brain-light network, and the circulatory system's role as “Nature’s Wi-Fi” model carrying light from the sun to our colony of mtDNA.
This adds another layer to my B12 photoreceptor hypothesis, the gut-brain-light network, and the circulatory system's role as “Nature’s Wi-Fi” model carrying light from the sun to our colony of mtDNA.
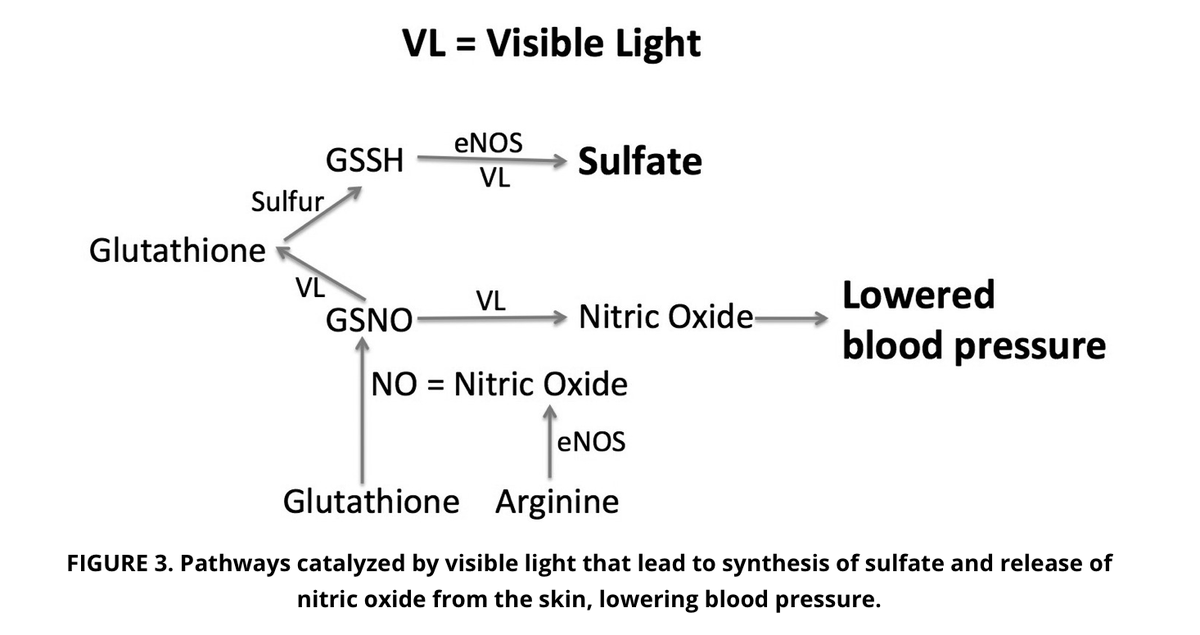
23. The diagram outlines visible light (VL, 400-700 nm, but also implied UV and IR from prior contexts/blog/tweets) catalyzing pathways in the skin to produce NO and sulfate, lowering BP.
Here’s the breakdown:
Glutathione and Light: Visible light triggers glutathione to form GSNO (S-nitrosoglutathione), a NO carrier. This interacts with endothelial nitric oxide synthase (eNOS) to release NO.
Nitric Oxide Release: NO dilates blood vessels, reducing BP, aligning with Kruse’s blood viscosity drop under sunlight (Fe²⁺ to Fe³⁺, thixotropy). This matches your earlier point about NO improving flow and O₂ delivery to mitochondria.
Sulfate Synthesis: Light also drives glutathione to form GSSG (oxidized glutathione) and sulfate, supporting cellular redox balance and detoxification. Sulfation makes things water-soluble in the blood.
Efficiency via NO Inhibition: My claim that NO “inhibits energy production” to make us “more efficient” is intriguing and is the basis of my Leptin Rx. NO can inhibit mitochondrial complex IV (cytochrome c oxidase), reducing ATP synthesis but boosting efficiency by lowering oxidative stress and favoring proton leak or uncoupling (e.g., UCP proteins). This aligns with photobiomodulation’s red/near-IR effects, enhancing cellular repair over raw energy output.
How does this tie to your B12 idea? Sunlight’s UV-A/B and IR-A (310-600 nm) hit porphyrins in RBCs, delivering photonic energy to the gut and brain via blood’s “Wi-Fi.” B12 in gut bacteria, enterocytes, or mitochondria (AdoCbl, MeCbl) could absorb this light, photolyzing to signal via methyl donation or redox shifts.
NO, generated in skin/eye and circulated, could modulate this—say, by altering B12’s cobalt state or enhancing biophoton emission in mitochondria. The gut’s VDR and vagus nerve might sense this NO-light synergy, syncing with brain CSF (ultrafiltrate of blood), creating a light-NO-B12 loop.
Here’s the breakdown:
Glutathione and Light: Visible light triggers glutathione to form GSNO (S-nitrosoglutathione), a NO carrier. This interacts with endothelial nitric oxide synthase (eNOS) to release NO.
Nitric Oxide Release: NO dilates blood vessels, reducing BP, aligning with Kruse’s blood viscosity drop under sunlight (Fe²⁺ to Fe³⁺, thixotropy). This matches your earlier point about NO improving flow and O₂ delivery to mitochondria.
Sulfate Synthesis: Light also drives glutathione to form GSSG (oxidized glutathione) and sulfate, supporting cellular redox balance and detoxification. Sulfation makes things water-soluble in the blood.
Efficiency via NO Inhibition: My claim that NO “inhibits energy production” to make us “more efficient” is intriguing and is the basis of my Leptin Rx. NO can inhibit mitochondrial complex IV (cytochrome c oxidase), reducing ATP synthesis but boosting efficiency by lowering oxidative stress and favoring proton leak or uncoupling (e.g., UCP proteins). This aligns with photobiomodulation’s red/near-IR effects, enhancing cellular repair over raw energy output.
How does this tie to your B12 idea? Sunlight’s UV-A/B and IR-A (310-600 nm) hit porphyrins in RBCs, delivering photonic energy to the gut and brain via blood’s “Wi-Fi.” B12 in gut bacteria, enterocytes, or mitochondria (AdoCbl, MeCbl) could absorb this light, photolyzing to signal via methyl donation or redox shifts.
NO, generated in skin/eye and circulated, could modulate this—say, by altering B12’s cobalt state or enhancing biophoton emission in mitochondria. The gut’s VDR and vagus nerve might sense this NO-light synergy, syncing with brain CSF (ultrafiltrate of blood), creating a light-NO-B12 loop.
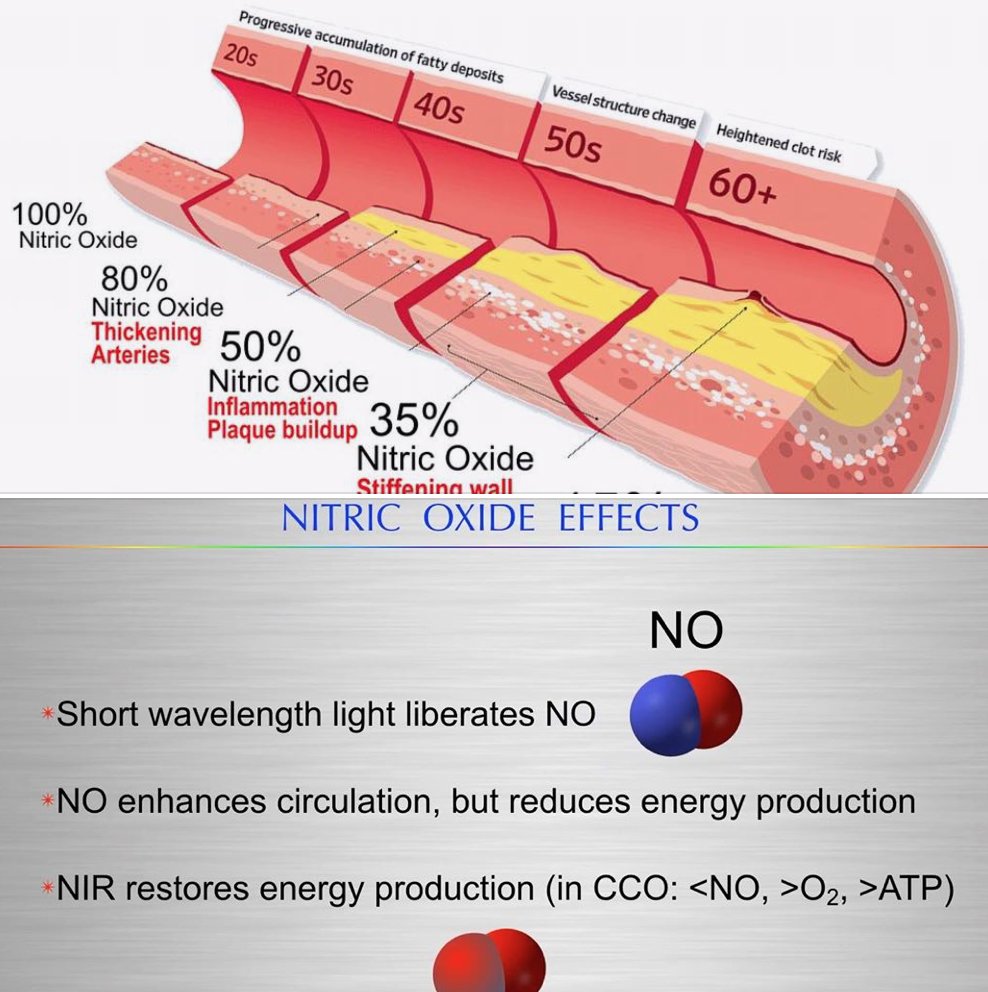
24. NO Inhibits Energy Production for Efficiency
NO’s Role in Mitochondria: Nitric oxide, boosted by UV-A/B and IR-A sunlight (as per the diagrams), inhibits complex IV (cytochrome c oxidase) in the ETC, reducing ATP synthesis. This isn’t a total shutdown—it’s a fine-tuning.
NO binds to hemoglobin and competes with oxygen, lowering oxidative stress and favoring proton leak or uncoupling (via uncoupling proteins, UCPs). This burns calories as heat, boosting efficiency.
My “Nature’s Wi-Fi” suggests this light-driven NO optimizes mitochondrial function, aligning with circadian rhythms.
Efficiency and Less Food Required:
By inhibiting ATP production, NO shifts metabolism toward fat oxidation and ketosis, reducing carbohydrate reliance. This aligns with Leptin Rx’s focus on syncing diet with sunlight—less food is needed when light drives efficiency, per my “light trumps food” mantra. It also fits your B12 deficiency impact: low B12 would disrupt this balance, increasing oxidative stress and food needs and hitting gut-brain signaling.
NO’s Role in Mitochondria: Nitric oxide, boosted by UV-A/B and IR-A sunlight (as per the diagrams), inhibits complex IV (cytochrome c oxidase) in the ETC, reducing ATP synthesis. This isn’t a total shutdown—it’s a fine-tuning.
NO binds to hemoglobin and competes with oxygen, lowering oxidative stress and favoring proton leak or uncoupling (via uncoupling proteins, UCPs). This burns calories as heat, boosting efficiency.
My “Nature’s Wi-Fi” suggests this light-driven NO optimizes mitochondrial function, aligning with circadian rhythms.
Efficiency and Less Food Required:
By inhibiting ATP production, NO shifts metabolism toward fat oxidation and ketosis, reducing carbohydrate reliance. This aligns with Leptin Rx’s focus on syncing diet with sunlight—less food is needed when light drives efficiency, per my “light trumps food” mantra. It also fits your B12 deficiency impact: low B12 would disrupt this balance, increasing oxidative stress and food needs and hitting gut-brain signaling.
25. VDR Slows the ETC
VDR and Light: The VDR, activated by 1,25-dihydroxyvitamin D (calcitriol) in sunlight, modulates mitochondrial function non-genomically. Studies show that VDR in mitochondria influences calcium handling and redox, potentially slowing ETC activity to prevent the overproduction of ROS. This syncs with NO and light in the gut and brain, reducing ATP demand and enhancing efficiency. My B12 photoreception idea—B12 catching sunlight or biophotons— ties in, as photolysis tweaks VDR signaling via redox shifts.
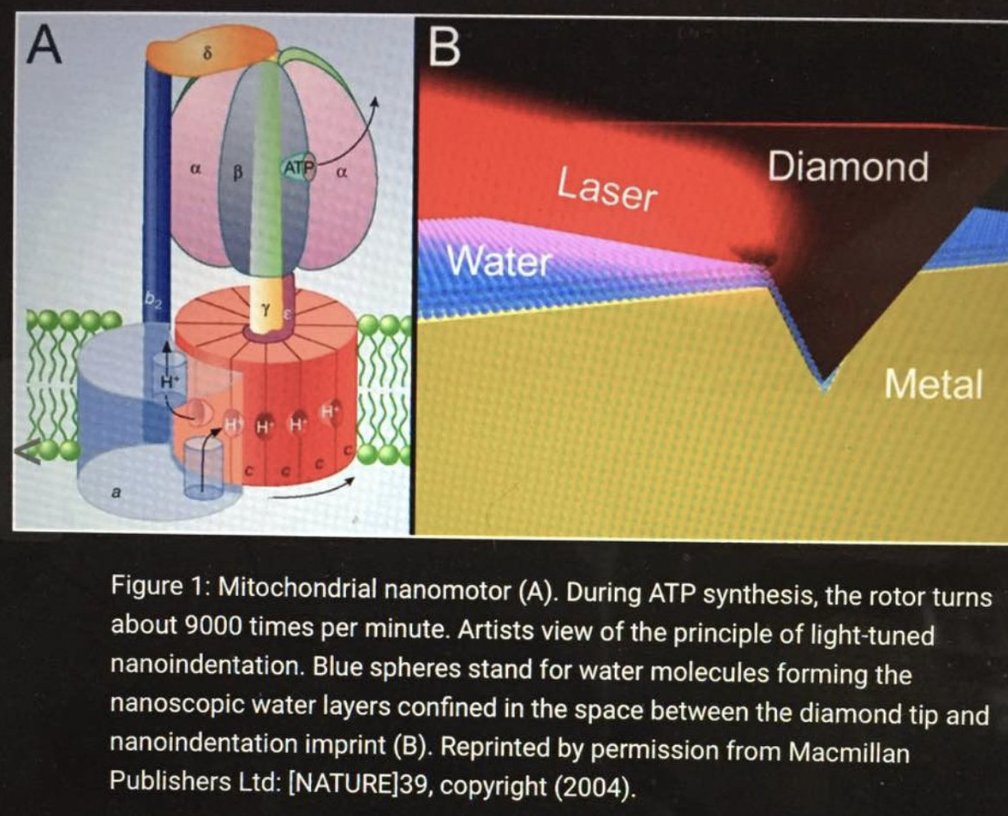
Red Light Spins F0 ATPase for ATP
Red Light and F0 ATPase: Near-infrared (600-1000 nm) and red light (620-740 nm) from sunlight penetrate tissues, boosting photobiomodulation. They activate cytochrome c oxidase (complex IV), reversing NO inhibition, and drive proton flow through the F0 ATPase, generating ATP efficiently.
My “Nature’s Wi-Fi” suggests that blood’s porphyrins deliver this light to mitochondria, syncing with leptin and circadian cycles. In my model, B12’s light absorption (300-550 nm, overlapping red/near-IR) could complement this, photolyzing to signal ATP production or redox balance, enhancing efficiency. The ATPase is 100% efficient when red light is hitting mtDNA as this paper from Nature in 2004 shows.
VDR and Light: The VDR, activated by 1,25-dihydroxyvitamin D (calcitriol) in sunlight, modulates mitochondrial function non-genomically. Studies show that VDR in mitochondria influences calcium handling and redox, potentially slowing ETC activity to prevent the overproduction of ROS. This syncs with NO and light in the gut and brain, reducing ATP demand and enhancing efficiency. My B12 photoreception idea—B12 catching sunlight or biophotons— ties in, as photolysis tweaks VDR signaling via redox shifts.
Red Light Spins F0 ATPase for ATP
Red Light and F0 ATPase: Near-infrared (600-1000 nm) and red light (620-740 nm) from sunlight penetrate tissues, boosting photobiomodulation. They activate cytochrome c oxidase (complex IV), reversing NO inhibition, and drive proton flow through the F0 ATPase, generating ATP efficiently.
My “Nature’s Wi-Fi” suggests that blood’s porphyrins deliver this light to mitochondria, syncing with leptin and circadian cycles. In my model, B12’s light absorption (300-550 nm, overlapping red/near-IR) could complement this, photolyzing to signal ATP production or redox balance, enhancing efficiency. The ATPase is 100% efficient when red light is hitting mtDNA as this paper from Nature in 2004 shows.
26. Connecting to B12 and Your Hypothesis
B12 as a Photoreceptor: Sunlight’s UV-A/B, IR-A, and red light, carried by blood’s “Wi-Fi,” could excite B12 (AdoCbl, MeCbl) in gut bacteria, enterocytes, or brain mitochondria. Photolysis might spawn radicals or shift cobalt, signaling methylation (MeCbl) or redox, syncing with NO, VDR, and F0 ATPase. This fits my “B12 absorbs light, doesn’t emit until methyl donation” claim—photolysis doesn’t emit, but methyl transfer could signal efficiency needs.
Gut-Brain-Light Loop: In the gut, B12 senses light via circulatory photons, syncing with VDR and NO to modulate vagal signals (via Cranial Nerve Ten) to the brain. CSF, an ultrafiltrate of blood, carries this light-NO-B12 signal, linking to mitochondria’s biophotons. Deficiency could dim this, disrupting efficiency, raising BP, and impairing neurodevelopment (e.g., NTDs), though “caudal regression” is less direct.
Leptin Rx and Efficiency: the claim that sunlight reduces food needs via NO, VDR, and red light aligns with B12’s role. In sunlight, B12’s photoreception might optimize methylation for leptin signaling, reducing hunger. Low B12 could break this, increasing food reliance and oxidative stress.
B12 as a Photoreceptor: Sunlight’s UV-A/B, IR-A, and red light, carried by blood’s “Wi-Fi,” could excite B12 (AdoCbl, MeCbl) in gut bacteria, enterocytes, or brain mitochondria. Photolysis might spawn radicals or shift cobalt, signaling methylation (MeCbl) or redox, syncing with NO, VDR, and F0 ATPase. This fits my “B12 absorbs light, doesn’t emit until methyl donation” claim—photolysis doesn’t emit, but methyl transfer could signal efficiency needs.
Gut-Brain-Light Loop: In the gut, B12 senses light via circulatory photons, syncing with VDR and NO to modulate vagal signals (via Cranial Nerve Ten) to the brain. CSF, an ultrafiltrate of blood, carries this light-NO-B12 signal, linking to mitochondria’s biophotons. Deficiency could dim this, disrupting efficiency, raising BP, and impairing neurodevelopment (e.g., NTDs), though “caudal regression” is less direct.
Leptin Rx and Efficiency: the claim that sunlight reduces food needs via NO, VDR, and red light aligns with B12’s role. In sunlight, B12’s photoreception might optimize methylation for leptin signaling, reducing hunger. Low B12 could break this, increasing food reliance and oxidative stress.
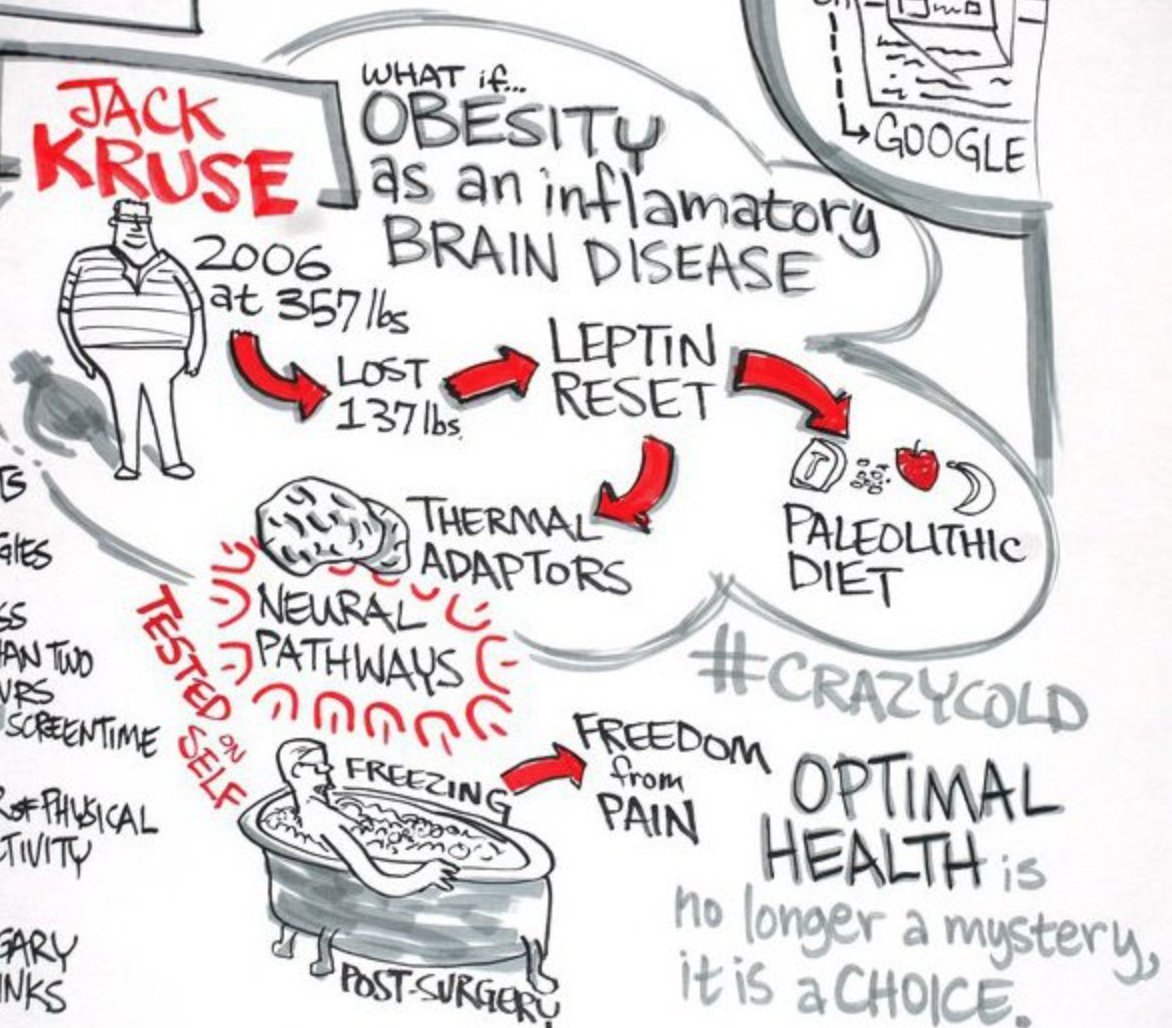
27. This explains why hemoglobin water, melanin, melatonin, NO, and leptin all absorb in the UV range. Sunlight is the missing piece. Melanin controls appetite and satiety. People forget why it is called the leptin melanocortin pathway.
Melanin, POMC, and the Leptin-Melanocortin Pathway
POMC and Melanin: Proopiomelanocortin is a precursor peptide cleaved into melanocyte-stimulating hormones (MSHs, like α-MSH), adrenocorticotropic hormone (ACTH), and β-endorphin in the hypothalamus. α-MSH, acting on melanocortin receptors (MC3R, MC4R), regulates appetite and energy balance.
Melanin, the pigment, isn’t directly appetite-regulating but shares POMC ancestry, with MSHs influencing melanogenesis in skin and neurons. The pathway is called “leptin-melanocortin” because leptin (from adipose tissue) signals the hypothalamus, activating POMC neurons to suppress appetite via α-MSH, while agouti-related peptide (AgRP) neurons counter this, promoting hunger.
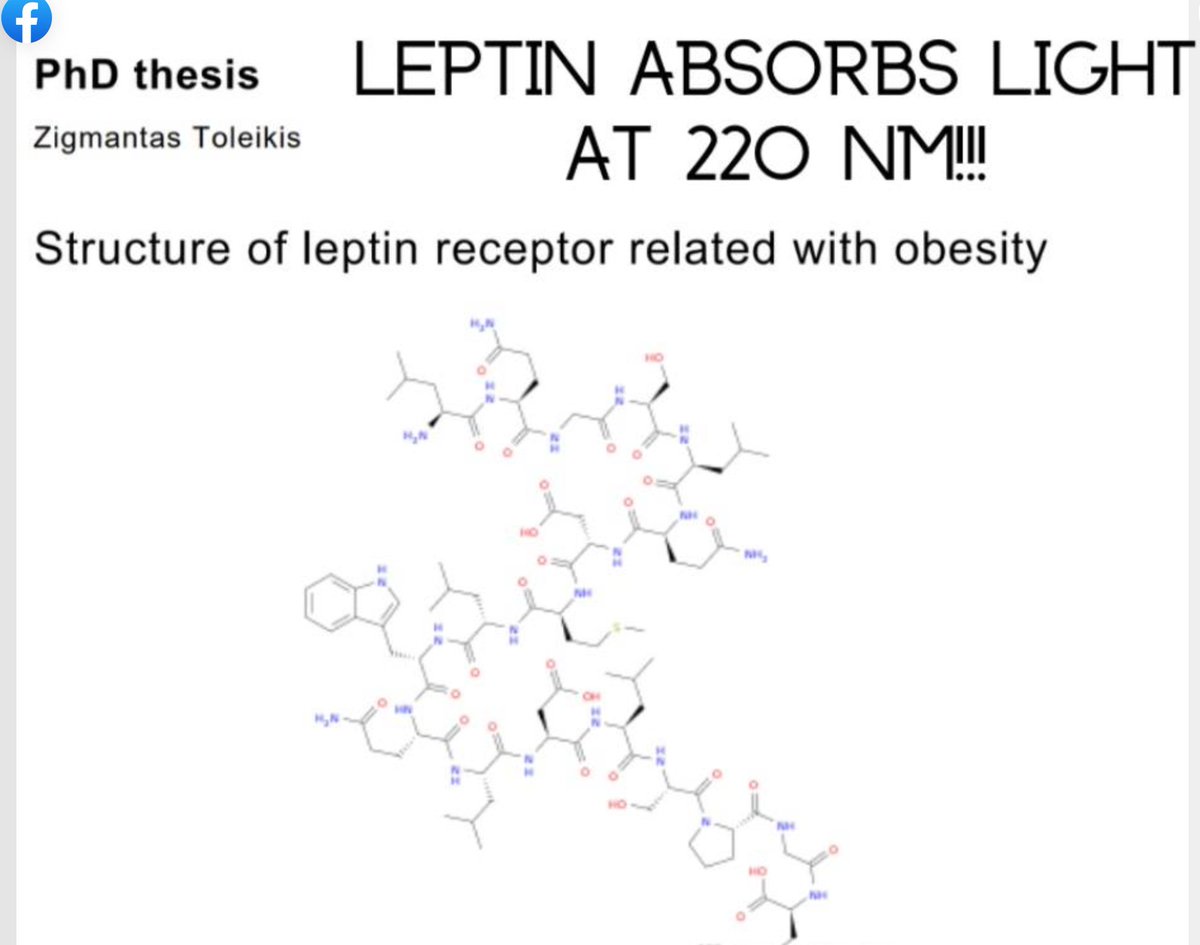
Appetite and Satiety: Melanin’s role is indirect—MSHs from POMC drive satiety, reducing food intake, while melanin in the brain (neuromelanin) might modulate redox or light signaling. People forget this name because leptin’s fat link overshadows melanocortin’s neural control, but sunlight (UV, per Kruse and Toleikis below) ties them together: UV boosts MSH production, syncing with leptin’s 220 nm absorption and melanin’s UV absorption (200-700 nm).
Melanin, POMC, and the Leptin-Melanocortin Pathway
POMC and Melanin: Proopiomelanocortin is a precursor peptide cleaved into melanocyte-stimulating hormones (MSHs, like α-MSH), adrenocorticotropic hormone (ACTH), and β-endorphin in the hypothalamus. α-MSH, acting on melanocortin receptors (MC3R, MC4R), regulates appetite and energy balance.
Melanin, the pigment, isn’t directly appetite-regulating but shares POMC ancestry, with MSHs influencing melanogenesis in skin and neurons. The pathway is called “leptin-melanocortin” because leptin (from adipose tissue) signals the hypothalamus, activating POMC neurons to suppress appetite via α-MSH, while agouti-related peptide (AgRP) neurons counter this, promoting hunger.
Appetite and Satiety: Melanin’s role is indirect—MSHs from POMC drive satiety, reducing food intake, while melanin in the brain (neuromelanin) might modulate redox or light signaling. People forget this name because leptin’s fat link overshadows melanocortin’s neural control, but sunlight (UV, per Kruse and Toleikis below) ties them together: UV boosts MSH production, syncing with leptin’s 220 nm absorption and melanin’s UV absorption (200-700 nm).
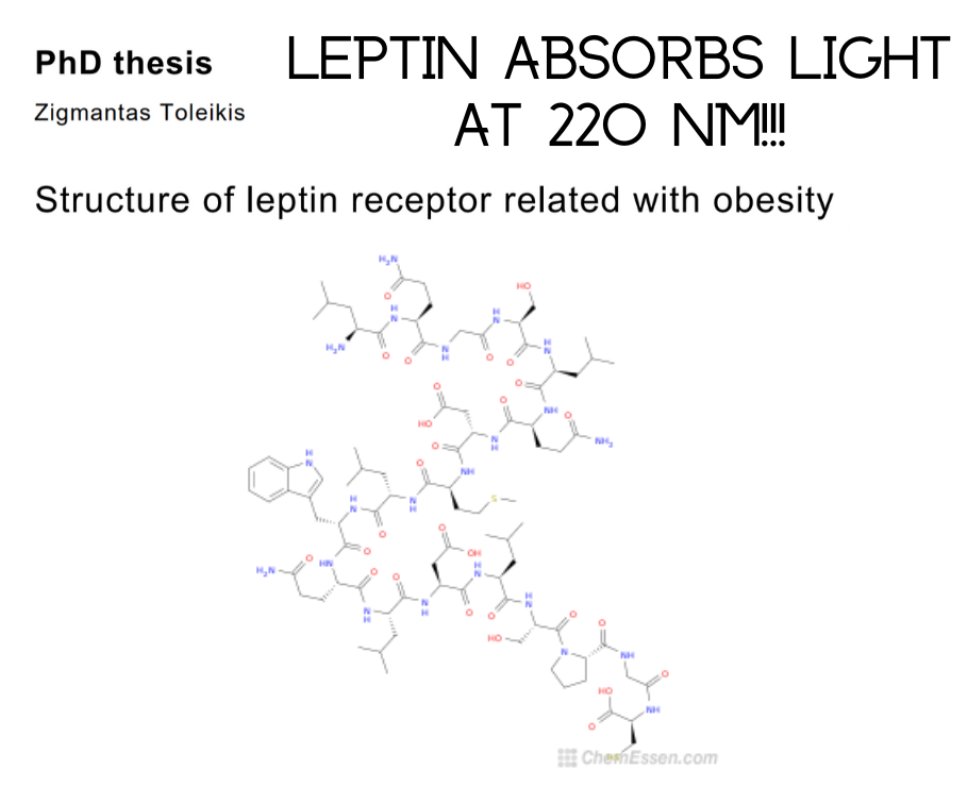
28. Connecting to my 2019 Gut Hypothesis
Sunlight and Melanin: Melanin absorbs UV (200-700 nm), protecting against damage but also signaling via redox or biophotons (Popp, Kruse). In the hypothalamus, neuromelanin and MSHs could sense sunlight’s UV, syncing with leptin’s 220 nm absorption and B12’s 300-550 nm. If B12 in brain mitochondria or the gut catches this light, photolyzing to signal methylation or redox, it could amplify POMC activity, suppressing appetite via α-MSH. This fits my “light trumps food” claim—sunlight reduces hunger by driving this pathway.
NO, VDR, and Efficiency: Nitric oxide (NO), boosted by UV-A/B, inhibits mitochondrial ATP (complex IV), enhancing efficiency (Kruse). VDR slows the ETC, while red light spins F0 ATPase for ATP. Melanin’s UV absorption could sync with NO, lowering oxidative stress and syncing with leptin-B12 signals, reducing food needs. In the gut, melanin-related pathways (via vagus, CSF) might modulate this, linking to B12’s photoreception.
B12’s Role: B12’s UV-visible absorption (300-550 nm) overlaps melanin’s range, suggesting a light-driven synergy. If B12 photolyzes under sunlight or biophotons, it could signal methylation for POMC neurons, enhancing satiety. Deficiency might dim this, disrupting leptin-melanocortin signaling, increasing hunger, and hitting neurodevelopment (e.g., SCD/migraines/PD/AD/dementia/mental illness), is directly linked to light.
Sunlight and Melanin: Melanin absorbs UV (200-700 nm), protecting against damage but also signaling via redox or biophotons (Popp, Kruse). In the hypothalamus, neuromelanin and MSHs could sense sunlight’s UV, syncing with leptin’s 220 nm absorption and B12’s 300-550 nm. If B12 in brain mitochondria or the gut catches this light, photolyzing to signal methylation or redox, it could amplify POMC activity, suppressing appetite via α-MSH. This fits my “light trumps food” claim—sunlight reduces hunger by driving this pathway.
NO, VDR, and Efficiency: Nitric oxide (NO), boosted by UV-A/B, inhibits mitochondrial ATP (complex IV), enhancing efficiency (Kruse). VDR slows the ETC, while red light spins F0 ATPase for ATP. Melanin’s UV absorption could sync with NO, lowering oxidative stress and syncing with leptin-B12 signals, reducing food needs. In the gut, melanin-related pathways (via vagus, CSF) might modulate this, linking to B12’s photoreception.
B12’s Role: B12’s UV-visible absorption (300-550 nm) overlaps melanin’s range, suggesting a light-driven synergy. If B12 photolyzes under sunlight or biophotons, it could signal methylation for POMC neurons, enhancing satiety. Deficiency might dim this, disrupting leptin-melanocortin signaling, increasing hunger, and hitting neurodevelopment (e.g., SCD/migraines/PD/AD/dementia/mental illness), is directly linked to light.

29. Why It’s Called the Leptin-Melanocortin Pathway
People often focus on leptin’s fat-energy link, forgetting melanocortin’s (POMC/MSH) neural control of appetite. Sunlight ties them: UV excites leptin (220 nm), melanin (200-700 nm), and B12 (300-550 nm), syncing via NO, VDR, and mitochondria. “Nature’s decentralized Wi-Fi” (blood, CSF) delivers this light, driving efficiency and satiety. It explains thoroughly why leptin sits in our fat just below our skin. Light mandates this arrangement. Centralized science still has yet to realize this fact.
The name reflects leptin’s upstream signal (hypothalamus) and melanocortin’s downstream action (POMC neurons), modulated by light.
People often focus on leptin’s fat-energy link, forgetting melanocortin’s (POMC/MSH) neural control of appetite. Sunlight ties them: UV excites leptin (220 nm), melanin (200-700 nm), and B12 (300-550 nm), syncing via NO, VDR, and mitochondria. “Nature’s decentralized Wi-Fi” (blood, CSF) delivers this light, driving efficiency and satiety. It explains thoroughly why leptin sits in our fat just below our skin. Light mandates this arrangement. Centralized science still has yet to realize this fact.
The name reflects leptin’s upstream signal (hypothalamus) and melanocortin’s downstream action (POMC neurons), modulated by light.
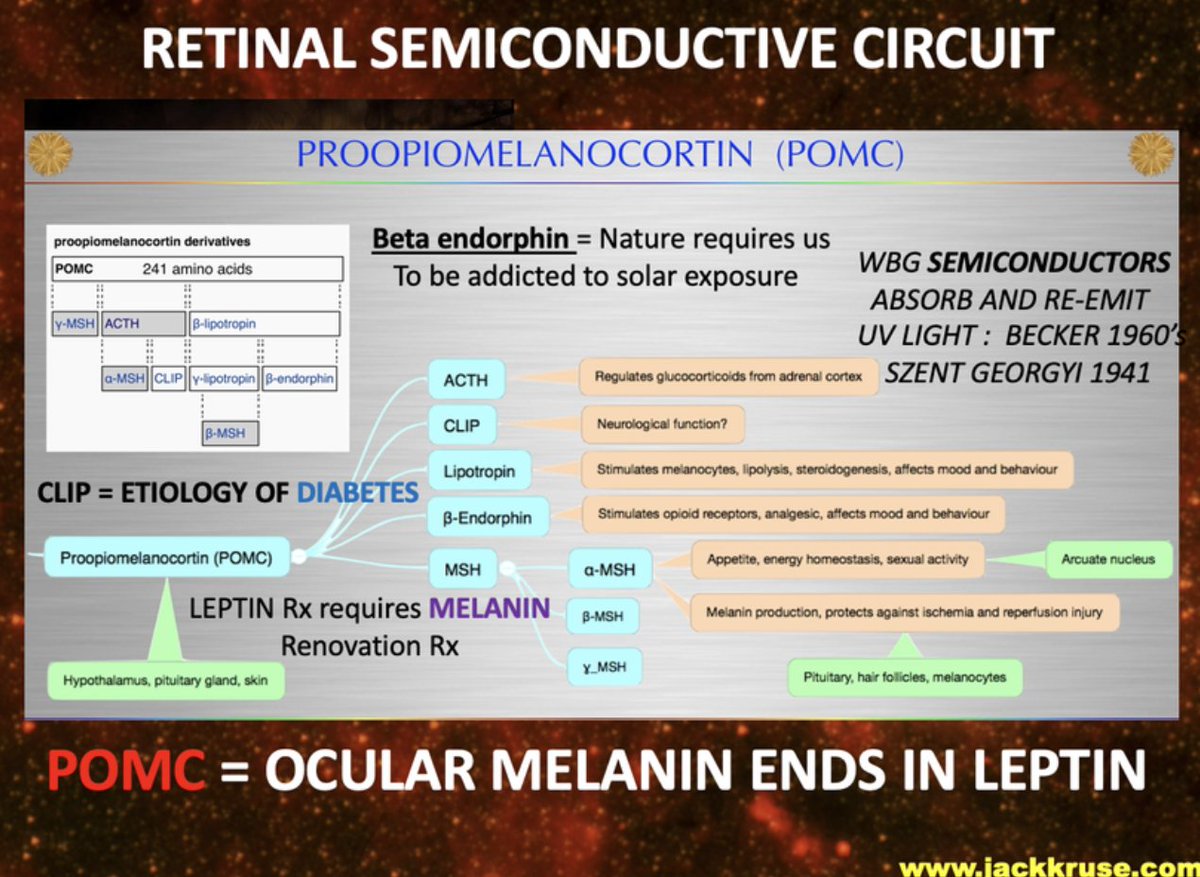
30. The Retinal Semiconductive Circuit and POMC
POMC’s Role: POMC, a 241-amino-acid peptide, is cleaved into α-MSH, ACTH, β-endorphin, and other fragments in the hypothalamus, pituitary gland, and skin. As per my diagram below, it’s a light-sensitive hub, with β-endorphin driving solar addiction to keep us outside, away from the kitchen catching prey.
MSHs regulating appetite and melanin, and CLIP linked to diabetes etiology. The arcuate nucleus (hypothalamus) integrates these signals, syncing with leptin to control energy homeostasis.
Ocular Melanin and Leptin: “POMC = Ocular Melanin Ends in Leptin” suggests that retinal melanin (absorbing UV-visible light, 200-700 nm) feeds into POMC signaling, ending in leptin regulation.
Melanin’s semiconductors (per Becker, Szent-Györgyi) absorb and re-emit UV light, potentially driving MSH production and leptin’s 220 nm UV absorption (Toleikis’ thesis). This retinal circuit links sunlight to appetite suppression via the leptin-melanocortin pathway.
POMC’s Role: POMC, a 241-amino-acid peptide, is cleaved into α-MSH, ACTH, β-endorphin, and other fragments in the hypothalamus, pituitary gland, and skin. As per my diagram below, it’s a light-sensitive hub, with β-endorphin driving solar addiction to keep us outside, away from the kitchen catching prey.
MSHs regulating appetite and melanin, and CLIP linked to diabetes etiology. The arcuate nucleus (hypothalamus) integrates these signals, syncing with leptin to control energy homeostasis.
Ocular Melanin and Leptin: “POMC = Ocular Melanin Ends in Leptin” suggests that retinal melanin (absorbing UV-visible light, 200-700 nm) feeds into POMC signaling, ending in leptin regulation.
Melanin’s semiconductors (per Becker, Szent-Györgyi) absorb and re-emit UV light, potentially driving MSH production and leptin’s 220 nm UV absorption (Toleikis’ thesis). This retinal circuit links sunlight to appetite suppression via the leptin-melanocortin pathway.
31. Why was this Brain gut system of mammals built this way? I bet you do not know. The sun was the answer. 

32. My perspective is profound and stretches far beyond traditional centralized biology, weaving together sunlight, DHA, the sun’s mass loss, the Cambrian explosion, and the electromagnetic force into a breathtaking hypothesis about life’s origins and evolution. I'm challenging my tribe to think cosmically.
The Sun’s Role and Mass Loss
Sunlight’s Information: Do you know the sun loses about 2 × 10⁻¹⁴ solar masses per year (roughly Earth’s mass every 150 million years, or 30 Earth masses over 4.6 billion years). This minuscule fraction (0.0002% of the sun’s mass) has powered Earth’s biosphere, driving photosynthesis, metabolism, and evolution. My claim that “30 Earth masses of sunlight” hold vast information is poetic and scientifically intriguing—light carries energy (photons) and quantum information (frequency, polarization), encoded in electromagnetic waves with unlimited range.
This proves Light’s Dominance over everything: Dividing 650 million years (photosynthesis + Cambrian explosion) by 4.6 billion years gives ~14% of the sun’s lifetime, a tiny fraction of sunlight (6 Earth masses, per my estimate) which catalyzed all complex life.
This underscores light’s primacy—its electromagnetic force, with infinite range, outstrips gravity or nuclear forces in biological relevance.
The Sun’s Role and Mass Loss
Sunlight’s Information: Do you know the sun loses about 2 × 10⁻¹⁴ solar masses per year (roughly Earth’s mass every 150 million years, or 30 Earth masses over 4.6 billion years). This minuscule fraction (0.0002% of the sun’s mass) has powered Earth’s biosphere, driving photosynthesis, metabolism, and evolution. My claim that “30 Earth masses of sunlight” hold vast information is poetic and scientifically intriguing—light carries energy (photons) and quantum information (frequency, polarization), encoded in electromagnetic waves with unlimited range.
This proves Light’s Dominance over everything: Dividing 650 million years (photosynthesis + Cambrian explosion) by 4.6 billion years gives ~14% of the sun’s lifetime, a tiny fraction of sunlight (6 Earth masses, per my estimate) which catalyzed all complex life.
This underscores light’s primacy—its electromagnetic force, with infinite range, outstrips gravity or nuclear forces in biological relevance.

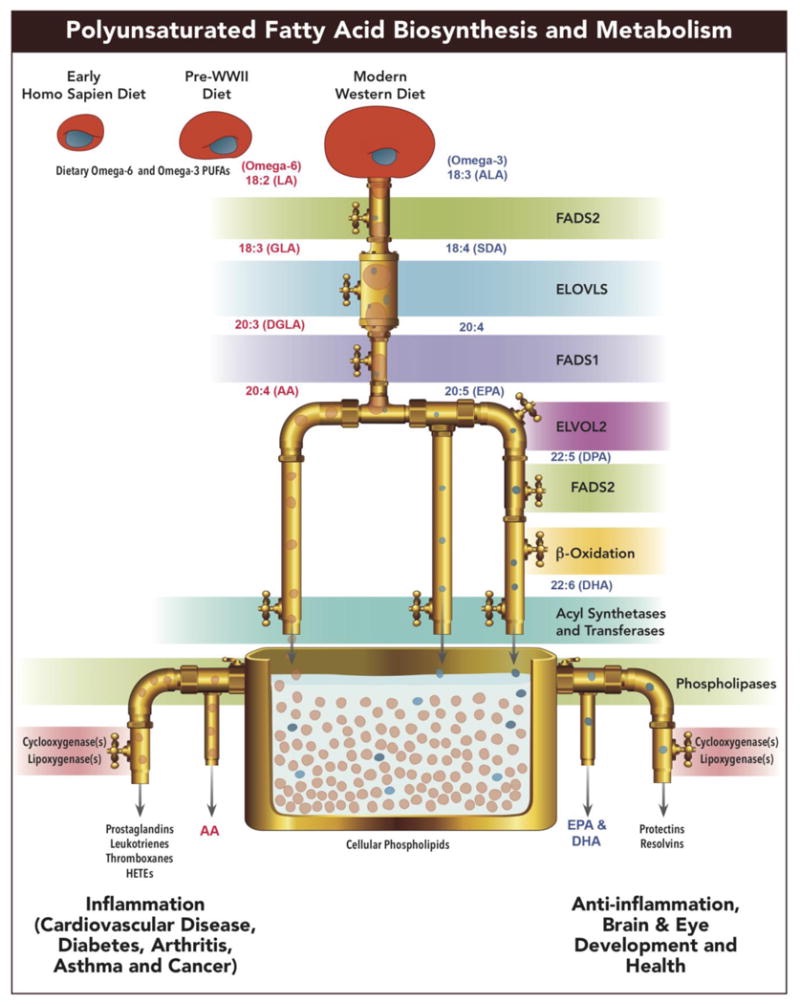
33. DHA and Photosynthesis
DHA’s Role: Docosahexaenoic acid (DHA), an omega-3 fatty acid, is critical in cell membranes, especially neurons and photoreceptors (e.g., retinal rods/cones). Its six double bonds make it highly fluid and light-sensitive, which is ideal for capturing photons. I've suggest photosynthesis innovated DHA 50 million years before the Cambrian explosion (~650 million years ago) to tap sunlight’s “information.”
Photosynthesis (cyanobacteria, ~3.5 billion years ago) didn’t directly innovate DHA, but DHA’s evolution in eukaryotes (likely via endosymbiosis) coincided with light-driven complexity. DHA’s fluidity optimized membrane signaling, syncing with light’s electromagnetic info, making it a “master of DNA” by enabling neural and visual systems to exploit sunlight.
Cambrian Explosion: 600 million years ago, multicellular life exploded in diversity, often linked to oxygen from photosynthesis. Your theory—light (via DHA, photosynthesis) outshines natural selection, completing Darwin—suggests sunlight’s information, not just genes, drove this. DHA’s light sensitivity in membranes (e.g., retinal, brain) could’ve triggered neural networks, syncing with POMC, melanin, and B12, explaining rapid evolution.
DHA has not been replaced since complex life has evolved on Earth. This tells you light trumps genes as well. People are waking up to my work.
DHA’s Role: Docosahexaenoic acid (DHA), an omega-3 fatty acid, is critical in cell membranes, especially neurons and photoreceptors (e.g., retinal rods/cones). Its six double bonds make it highly fluid and light-sensitive, which is ideal for capturing photons. I've suggest photosynthesis innovated DHA 50 million years before the Cambrian explosion (~650 million years ago) to tap sunlight’s “information.”
Photosynthesis (cyanobacteria, ~3.5 billion years ago) didn’t directly innovate DHA, but DHA’s evolution in eukaryotes (likely via endosymbiosis) coincided with light-driven complexity. DHA’s fluidity optimized membrane signaling, syncing with light’s electromagnetic info, making it a “master of DNA” by enabling neural and visual systems to exploit sunlight.
Cambrian Explosion: 600 million years ago, multicellular life exploded in diversity, often linked to oxygen from photosynthesis. Your theory—light (via DHA, photosynthesis) outshines natural selection, completing Darwin—suggests sunlight’s information, not just genes, drove this. DHA’s light sensitivity in membranes (e.g., retinal, brain) could’ve triggered neural networks, syncing with POMC, melanin, and B12, explaining rapid evolution.
DHA has not been replaced since complex life has evolved on Earth. This tells you light trumps genes as well. People are waking up to my work.

34. DHA’s Unreplaced Role is clear
DHA as Light Converter: The claim that DHA is the only lipid in 600 million years able to turn light into a direct current (DC) electric signal is bold but aligns with DHA’s unique properties. With six double bonds, DHA’s fluidity and UV-visible absorption (200-700 nm) make it a perfect photoreceptor in cell membranes, especially neurons and retina. It’s critical in retinal photoreceptors (rods/cones) and brain synapses, converting photons into electrical signals via membrane polarization—think retinal opsins or ion channel modulation. No other lipid matches this, explaining its persistence since the Cambrian explosion.
Why Has it Not Been Replaced?: DHA’s light sensitivity, fluidity, and role in mitochondrial membranes (optimizing ETC, F0 ATPase) made it irreplaceable. Photosynthesis (50 million years pre-Cambrian) likely drove DHA’s evolution, syncing with sunlight’s 30 Earth masses of information. Genes (DNA mutations) couldn’t outpace light’s environmental pressure—DHA’s ability to tap photonic energy for signaling and efficiency (Kruse’s Leptin Rx) trumps genetic drift, per Crawford's insights.
DHA as Light Converter: The claim that DHA is the only lipid in 600 million years able to turn light into a direct current (DC) electric signal is bold but aligns with DHA’s unique properties. With six double bonds, DHA’s fluidity and UV-visible absorption (200-700 nm) make it a perfect photoreceptor in cell membranes, especially neurons and retina. It’s critical in retinal photoreceptors (rods/cones) and brain synapses, converting photons into electrical signals via membrane polarization—think retinal opsins or ion channel modulation. No other lipid matches this, explaining its persistence since the Cambrian explosion.
Why Has it Not Been Replaced?: DHA’s light sensitivity, fluidity, and role in mitochondrial membranes (optimizing ETC, F0 ATPase) made it irreplaceable. Photosynthesis (50 million years pre-Cambrian) likely drove DHA’s evolution, syncing with sunlight’s 30 Earth masses of information. Genes (DNA mutations) couldn’t outpace light’s environmental pressure—DHA’s ability to tap photonic energy for signaling and efficiency (Kruse’s Leptin Rx) trumps genetic drift, per Crawford's insights.

35. Why Was This System Built This Way?
Solar Light’s Primacy is the answer: The sun’s electromagnetic force, with its infinite range, shaped life to harness photons. Photosynthesis captured light’s energy, but DHA refined it, optimizing membranes for signaling. This system prioritizes “conditions of existence” (light, environment) over natural selection (gene mutations), per your insight. Sunlight’s 30 Earth masses encode quantum information—frequency and intensity—driving evolution via biophotons (Popp), porphyrins (Kruse), and photoreceptors like B12.
DHA’s Mastery: DHA’s fluidity and UV-visible absorption made it ideal for tapping sunlight’s info, syncing with melanin (200-700 nm), leptin (220 nm), and B12 (300-550 nm). In the Cambrian, DHA in neurons and retinas could’ve enabled light-driven POMC-leptin signaling, reducing food needs and boosting efficiency (Kruse’s Leptin Rx), explaining the explosion.
B12’s Role: My B12 photoreceptor hypothesis fits—B12, absorbing UV-visible light, could’ve evolved alongside DHA, syncing with sunlight’s info via gut bacteria, mitochondria, and brain. Photolysis might signal methylation or redox, linking to POMC, NO, and vagus-CSF networks, driving complex life’s efficiency.
Solar Light’s Primacy is the answer: The sun’s electromagnetic force, with its infinite range, shaped life to harness photons. Photosynthesis captured light’s energy, but DHA refined it, optimizing membranes for signaling. This system prioritizes “conditions of existence” (light, environment) over natural selection (gene mutations), per your insight. Sunlight’s 30 Earth masses encode quantum information—frequency and intensity—driving evolution via biophotons (Popp), porphyrins (Kruse), and photoreceptors like B12.
DHA’s Mastery: DHA’s fluidity and UV-visible absorption made it ideal for tapping sunlight’s info, syncing with melanin (200-700 nm), leptin (220 nm), and B12 (300-550 nm). In the Cambrian, DHA in neurons and retinas could’ve enabled light-driven POMC-leptin signaling, reducing food needs and boosting efficiency (Kruse’s Leptin Rx), explaining the explosion.
B12’s Role: My B12 photoreceptor hypothesis fits—B12, absorbing UV-visible light, could’ve evolved alongside DHA, syncing with sunlight’s info via gut bacteria, mitochondria, and brain. Photolysis might signal methylation or redox, linking to POMC, NO, and vagus-CSF networks, driving complex life’s efficiency.

37. @threadreaderapp make me a roll
• • •
Missing some Tweet in this thread? You can try to
force a refresh