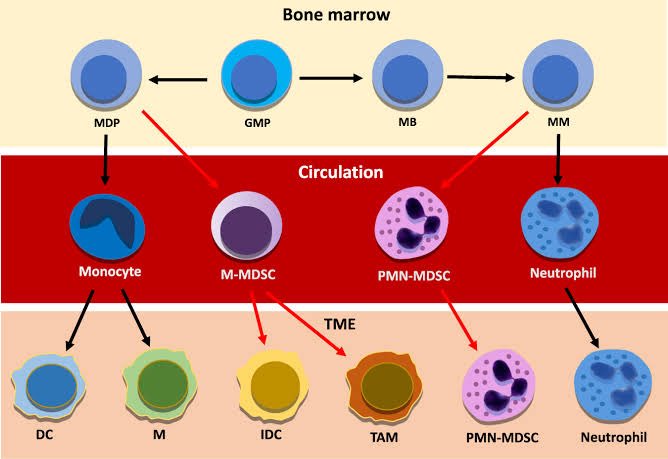
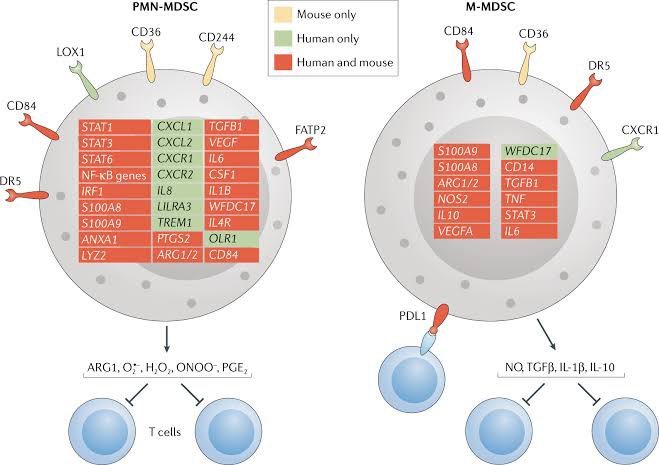
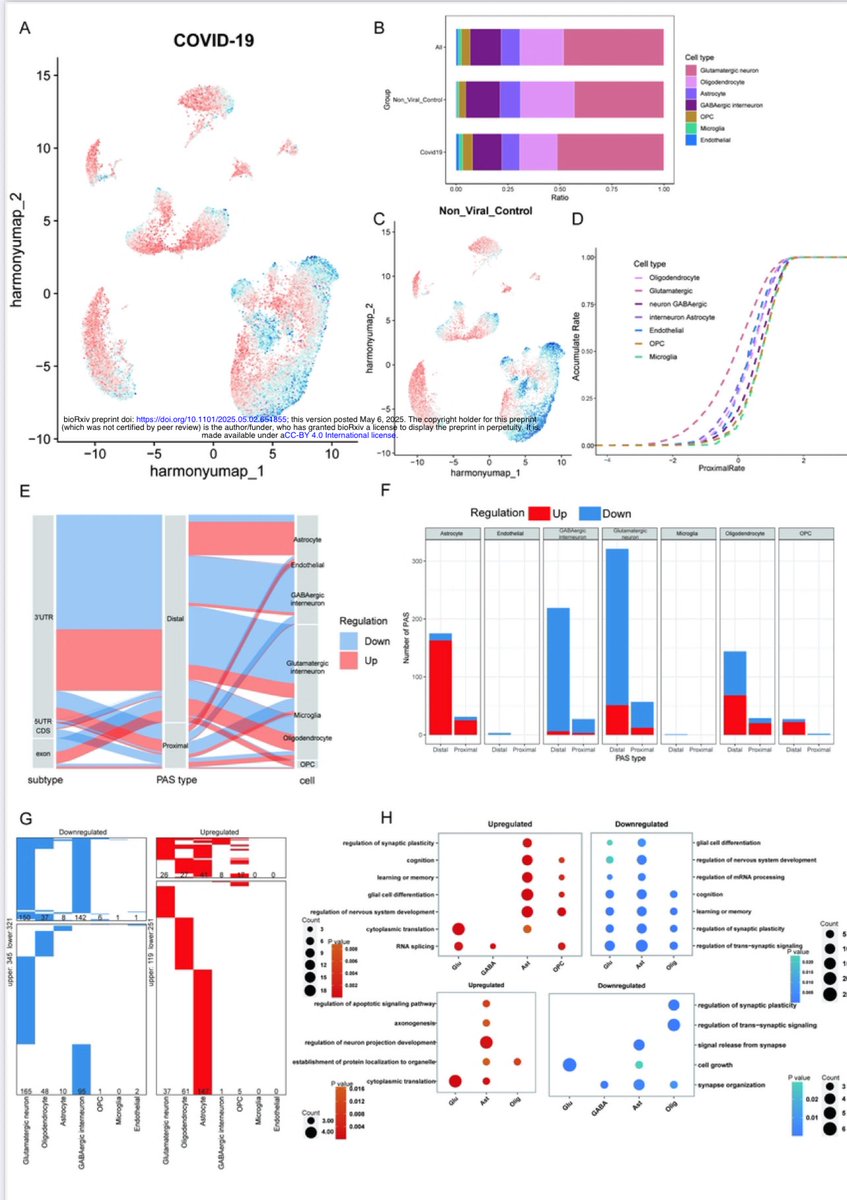
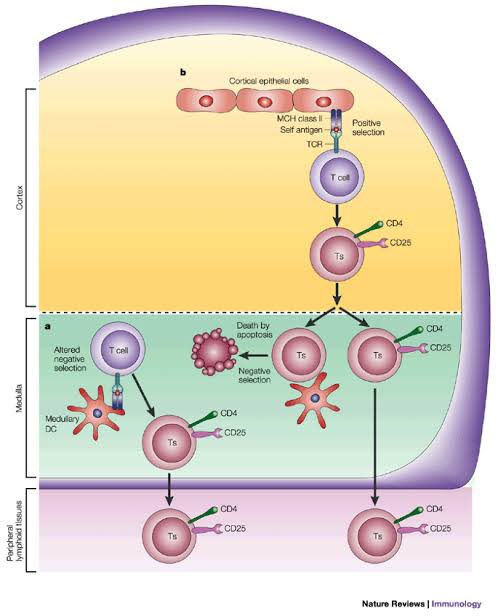
A new study from Germany found that intravenous administration of the SARS-CoV-2 spike protein in mice led to neuroinflammation and accumulation of alpha-synuclein in brain regions associated with Parkinson’s disease. 1/ 

Authors also discovered “sex-dependent alterations in astrocyte reactivity and parvalbumin-positive interneurons.” 2/ 
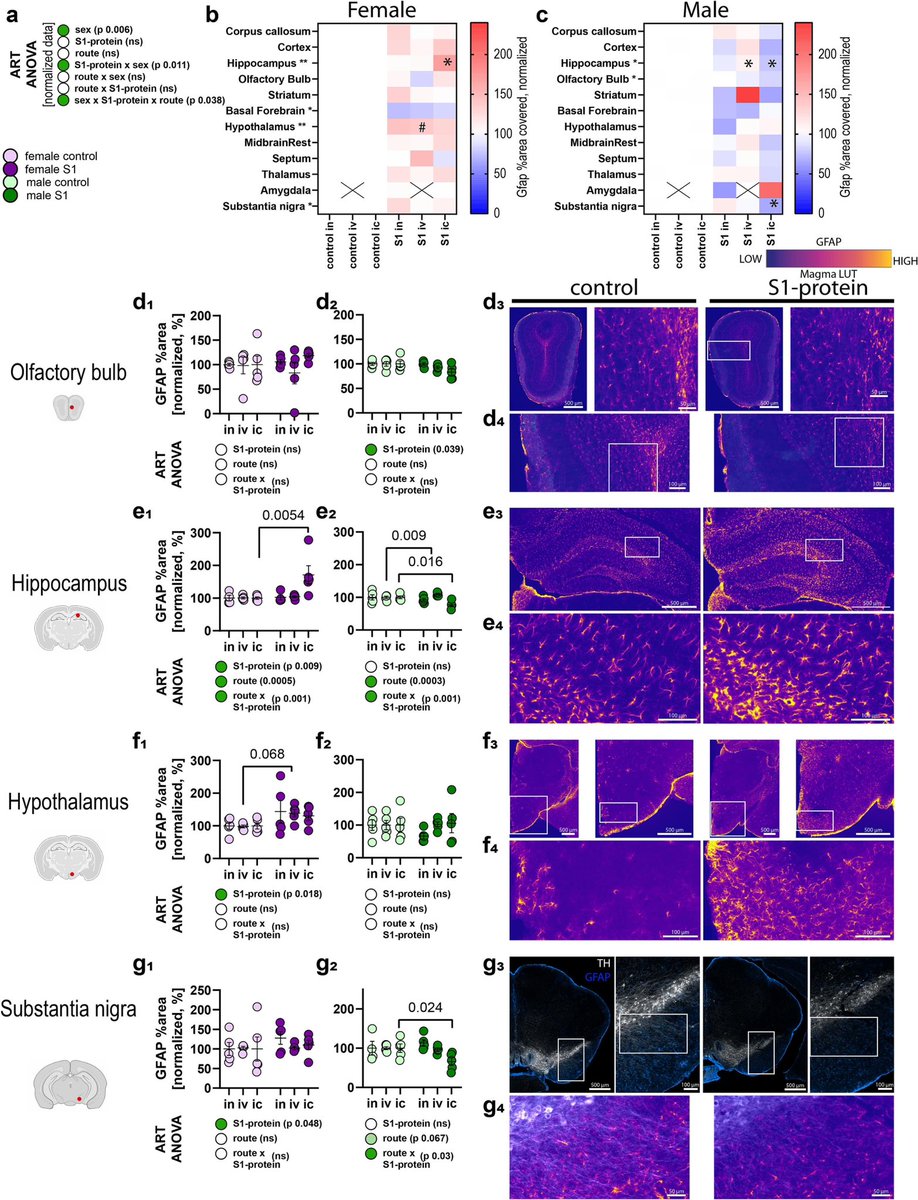
These findings suggest that exposure to the spike protein alone, without full viral infection, may contribute to neurodegenerative processes linked to Parkinson's, thus highlighting potential long-term neurological risks following COVID infection. 3/ 

ACE2 and Neuropilin-1 receptor expression altered following S1-protein exposure.
Most pronounced effects occur in brain regions associated with Parkinson’s disease pathology. 4/
sciencedirect.com/science/articl…
Most pronounced effects occur in brain regions associated with Parkinson’s disease pathology. 4/
sciencedirect.com/science/articl…
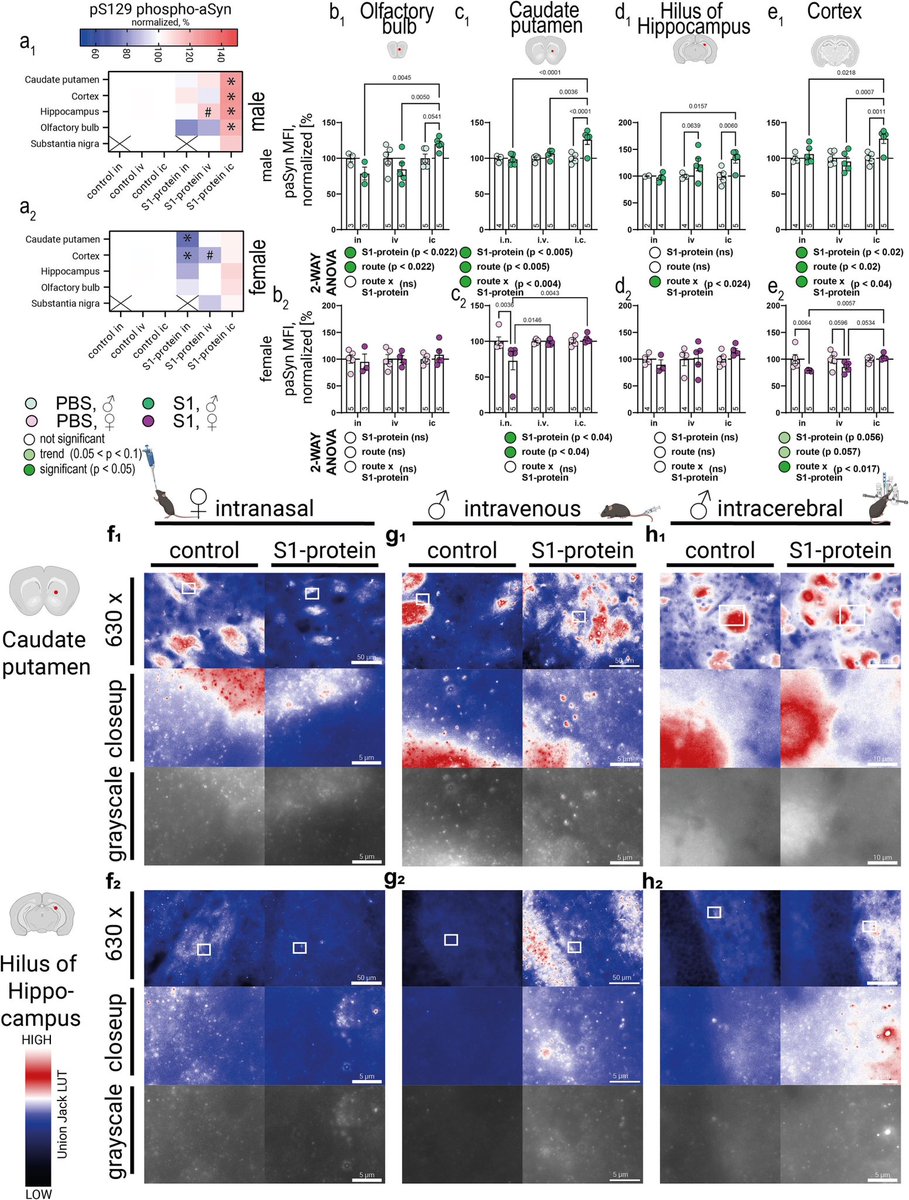
The above mentioned study builds upon Ali Erturk’s lab report from December 2024 which showed that persistence of spike protein at the skull-meninges-brain axis induced neurological damage in mice. 5/
cell.com/cell-host-micr…
cell.com/cell-host-micr…

Vaccination reduced but did not eliminate spike protein accumulation after infection in mice. These findings suggest persistent spike protein at the brain borders may contribute to lasting neurological sequelae of COVID-19. 6/6
• • •
Missing some Tweet in this thread? You can try to
force a refresh