$NWBO
1/
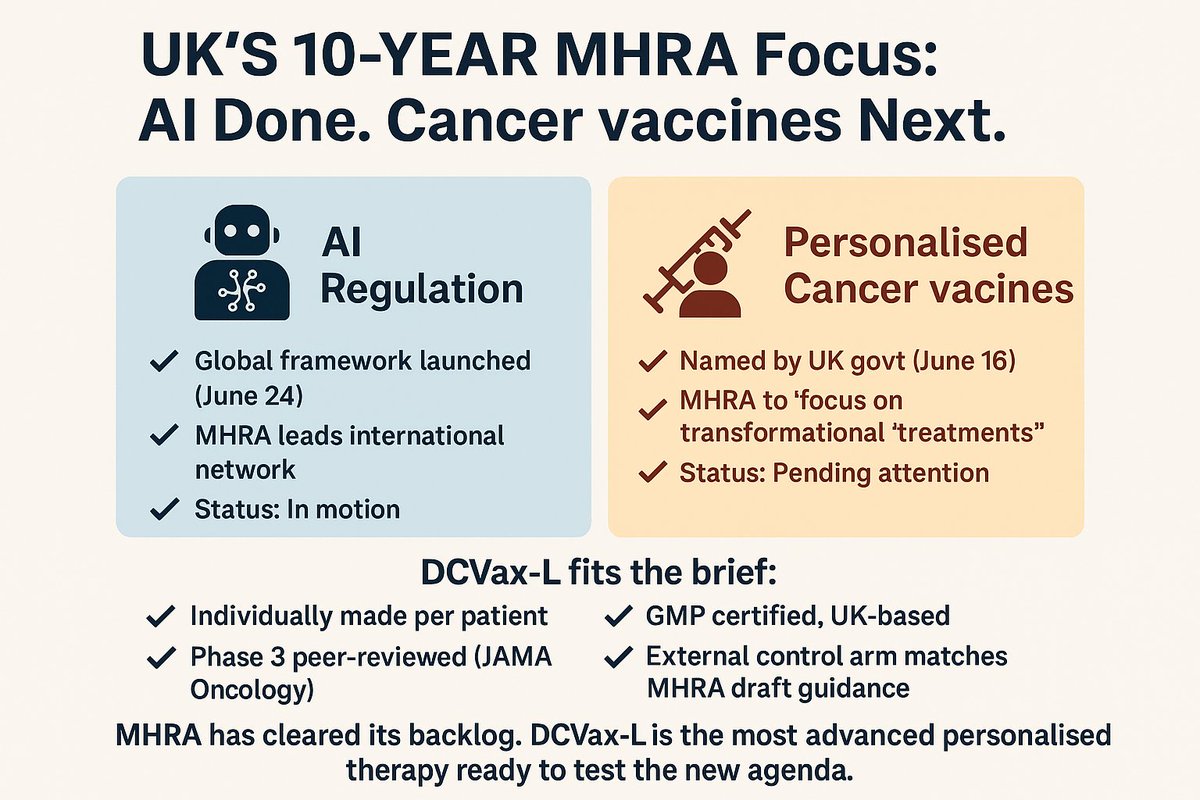
Has the MHRA’s new 2025 guidance on external controls anything to do with NWBOs MHRA application?
Let’s examine that claim from here
using the ACTUAL verbatim language from the MHRA guideline. investorshub.advfn.com/boards/read_ms…
1/
Has the MHRA’s new 2025 guidance on external controls anything to do with NWBOs MHRA application?
Let’s examine that claim from here
using the ACTUAL verbatim language from the MHRA guideline. investorshub.advfn.com/boards/read_ms…

The MHRA guidance is titled:
“Use of External Control Arms Based on Real-World Data to Support Regulatory Decisions”
It focuses on ECAs in situations where randomization is infeasible or unethical.
NWBO’s DCVax-L trial fits that exact profile.
“Use of External Control Arms Based on Real-World Data to Support Regulatory Decisions”
It focuses on ECAs in situations where randomization is infeasible or unethical.
NWBO’s DCVax-L trial fits that exact profile.
3/
“While the guideline is specifically aimed at sponsors planning to use RWD ECAs, many of the general principles would be relevant for external controls drawn from other sources, such as previously completed clinical trials.”
Covers trial-based comparators like DCVax-L’s.
“While the guideline is specifically aimed at sponsors planning to use RWD ECAs, many of the general principles would be relevant for external controls drawn from other sources, such as previously completed clinical trials.”
Covers trial-based comparators like DCVax-L’s.
4/
“The ECA can be either prospective, based on data collected contemporaneously to the clinical trial, or a historical control based on existing data.”
NWBO used historical control data from 5 large GBM trials. That’s within scope.
“The ECA can be either prospective, based on data collected contemporaneously to the clinical trial, or a historical control based on existing data.”
NWBO used historical control data from 5 large GBM trials. That’s within scope.
5/
“Therefore, there is no general scenario where the use of RWD external controls is explicitly ruled out.”
So MHRA is signaling flexibility.
No sign on restriction for trials like DCVax-L.
“Therefore, there is no general scenario where the use of RWD external controls is explicitly ruled out.”
So MHRA is signaling flexibility.
No sign on restriction for trials like DCVax-L.
6/
NWBO had full individual patient-level data (IPD) for its own arm, and used Matching-Adjusted Indirect Comparison (MAIC) to align with published trial comparators.
That’s fully aligned with MHRA’s expectations for matching methods and transparency.
NWBO had full individual patient-level data (IPD) for its own arm, and used Matching-Adjusted Indirect Comparison (MAIC) to align with published trial comparators.
That’s fully aligned with MHRA’s expectations for matching methods and transparency.
7/
Also important:
In 2022, MHRA approved NWBO’s Pediatric Investigation Plan (PIP) using the same external control methodology as the adult DCVax-L trial.
That approval predates the APPGBT report and this 2025 guidance.
Also important:
In 2022, MHRA approved NWBO’s Pediatric Investigation Plan (PIP) using the same external control methodology as the adult DCVax-L trial.
That approval predates the APPGBT report and this 2025 guidance.
The APPGBT’s 2023 report calls explicitly for regulators to adopt the external control model - using DCVax-L as its working precedent.
MHRA’s 2025 guidance reflects that shift in policy already operationalized.
MHRA’s 2025 guidance reflects that shift in policy already operationalized.
9/
This is a matter of reading the MHRA guidance carefully and recognizing how it aligns with the DCVax-L trial design - one the MHRA has already reviewed and accepted from NWBO.
This is a matter of reading the MHRA guidance carefully and recognizing how it aligns with the DCVax-L trial design - one the MHRA has already reviewed and accepted from NWBO.
10/
The guidance CONFIRMS that the trial design used by NWBO - based on matched external controls from prior studies - is fully supported under the MHRA’s current regulatory framework.
EVERYTHING TO DO WITH NWBO.
The full walkthrough in this PDF:
drive.google.com/file/d/1liOKGg…
The guidance CONFIRMS that the trial design used by NWBO - based on matched external controls from prior studies - is fully supported under the MHRA’s current regulatory framework.
EVERYTHING TO DO WITH NWBO.
The full walkthrough in this PDF:
drive.google.com/file/d/1liOKGg…

• • •
Missing some Tweet in this thread? You can try to
force a refresh