
How to get URL link on X (Twitter) App




https://twitter.com/peter_brit/status/1940794065269694700
 1/
1/ 

 The MHRA guidance is titled:
The MHRA guidance is titled:
 2/
2/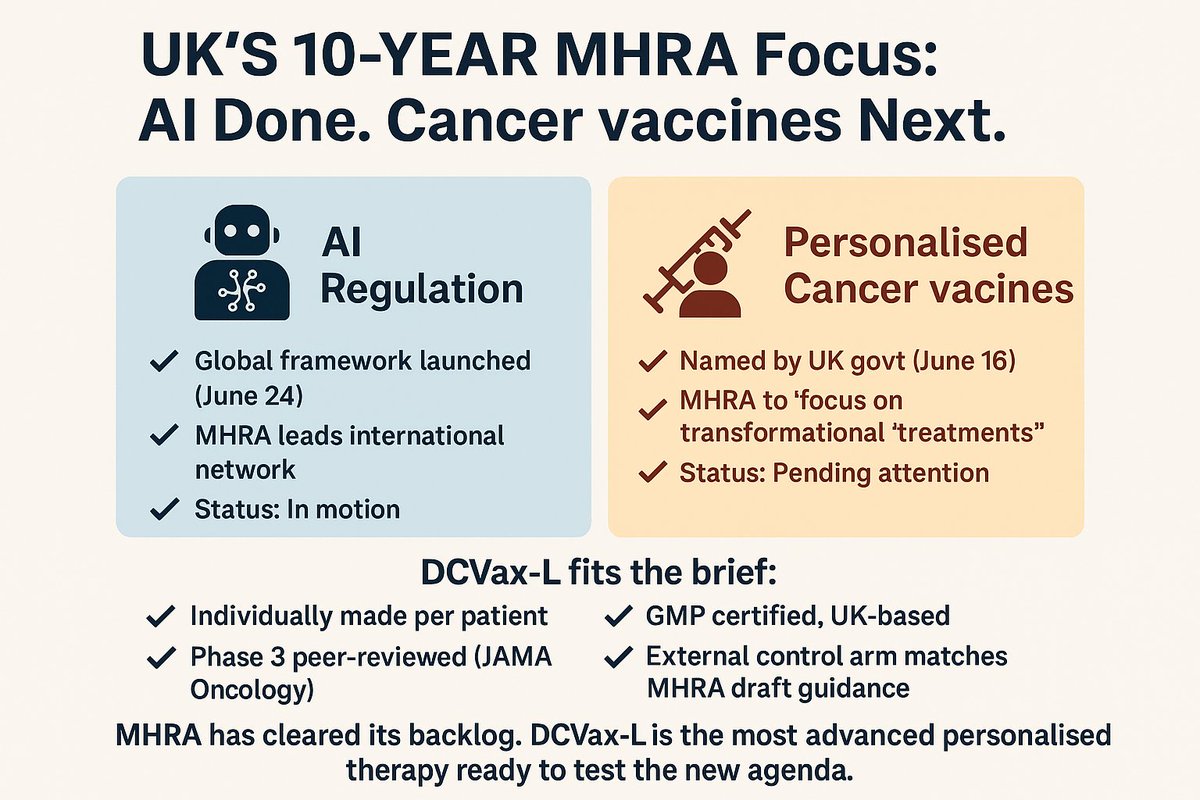
 June 16:
June 16:

 2/
2/
 1/
1/
 2/
2/
https://twitter.com/HenryMuney/status/1925273886788395270
 2/11
2/11
 2/9
2/9

 2/13
2/13

 2/8
2/8
 1/11 🚀
1/11 🚀

 1/18
1/18

 Please follow @d_stock07734 for additional due diligence regarding the Czech Trial results and other possible links.
Please follow @d_stock07734 for additional due diligence regarding the Czech Trial results and other possible links.https://x.com/d_stock07734/status/1919062992500543831


 2/
2/