𝗣𝗔𝗥𝗞𝗜𝗡𝗦𝗢𝗡’𝗦 & 𝗟𝗜𝗚𝗛𝗧 – 𝗔𝗡 𝗨𝗡𝗧𝗢𝗟𝗗 𝗦𝗧𝗢𝗥𝗬
A mega thread on how sunlight, circadian rhythms, and red light therapy protect the brain and improve Parkinson’s symptoms.
I have linked over 50 studies.
🧵

A mega thread on how sunlight, circadian rhythms, and red light therapy protect the brain and improve Parkinson’s symptoms.
I have linked over 50 studies.
🧵
I wrote this thread because I have a client who has seen massive benefits from sunlight and photobiomodulation for helping manage his Parkinson's disease symptoms.
*𝘿𝙞𝙨𝙘𝙡𝙖𝙞𝙢𝙚𝙧: 𝙉𝙤𝙣𝙚 𝙤𝙛 𝙩𝙝𝙞𝙨 𝙞𝙨 𝙢𝙚𝙙𝙞𝙘𝙖𝙡 𝙖𝙙𝙫𝙞𝙘𝙚*
*𝘿𝙞𝙨𝙘𝙡𝙖𝙞𝙢𝙚𝙧: 𝙉𝙤𝙣𝙚 𝙤𝙛 𝙩𝙝𝙞𝙨 𝙞𝙨 𝙢𝙚𝙙𝙞𝙘𝙖𝙡 𝙖𝙙𝙫𝙞𝙘𝙚*
𝗦𝘂𝗺𝗺𝗮𝗿𝘆 - We will be covering a lot of topics!
1) Interaction Between Light & the Body
2) Light & The Human Brain
3) Sunlight & Parkinson's Disease (PD)
4) Light Therapy & PD
5) Vitamin D & PD
6) Near Infrared Light & PD
7) Artificial Light & PD
8) Light, Pain & PD
9) Circadian Rhythms & PD
1) Interaction Between Light & the Body
2) Light & The Human Brain
3) Sunlight & Parkinson's Disease (PD)
4) Light Therapy & PD
5) Vitamin D & PD
6) Near Infrared Light & PD
7) Artificial Light & PD
8) Light, Pain & PD
9) Circadian Rhythms & PD
Sunlight is one of the oldest selective pressures under which life has evolved.
For aeons, the sun has stood as the foremost external influence shaping living organisms, an enduring presence since the dawn of life itself.
For aeons, the sun has stood as the foremost external influence shaping living organisms, an enduring presence since the dawn of life itself.
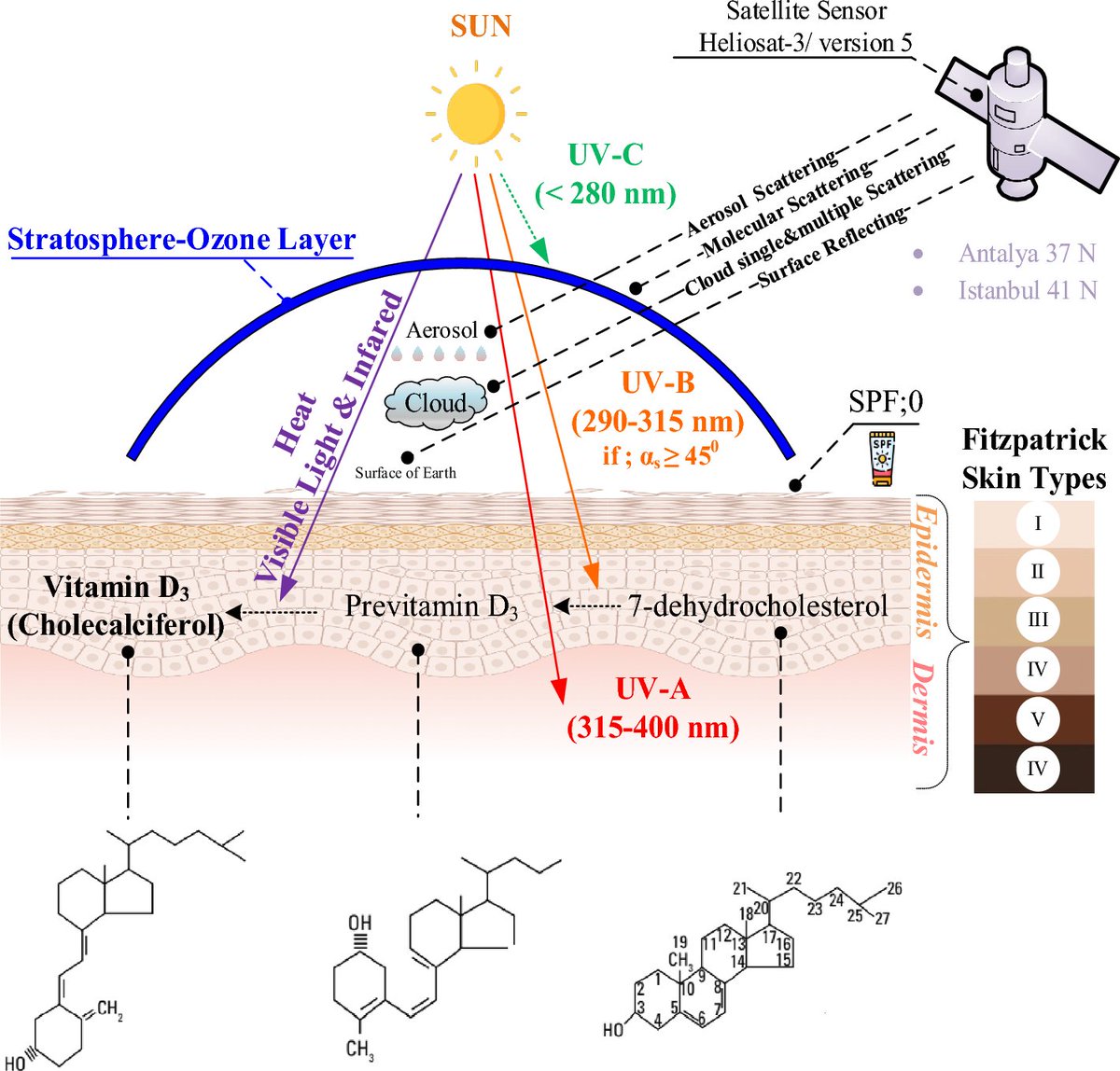
Considering the unique optics of the human body, and the number of known chromophores and biological processes which respond to specific wavelengths of sunlight, it should come as no surprise that sunlight may whiplay a role in human health.
A lifetime of sunlight exposure shapes your risk for conditions like depression, diabetes, Alzheimer’s & Parkinson’s too.
https://x.com/sam_soete/status/1808909967035875565
First lets discuss the conventional approach to PD and why it is failing you.
The conventional treatment for PD is dopamine replacement therapy (DART) in the form of levodopa (L-DOPA).
The conventional treatment for PD is dopamine replacement therapy (DART) in the form of levodopa (L-DOPA).
While DART initially addresses dopamine deficiency in the nigrostriatal system, prolonged use leads to issues like drug tolerance and side-effects.
The average effective treatment duration is 2 to 5 years, after which escalating doses cause severe side effects such as psychosis, hyperkinesia/dyskinesia, dementia, and dopamine dysregulation syndrome(1).
The average effective treatment duration is 2 to 5 years, after which escalating doses cause severe side effects such as psychosis, hyperkinesia/dyskinesia, dementia, and dopamine dysregulation syndrome(1).
Resulting polypharmacy is a major problem and significantly affects quality of life. Indeed, in some cases “the treatment becomes worse than the disease itself”(2).
However, the scientific literature (actually Nature) may offer an alternative or adjunctive therapy in the form of light that could aid those with PD.
In order to understand the role that light can play, we have to dig into the interactions between light & the body.
𝗜𝗻𝘁𝗲𝗿𝗮𝗰𝘁𝗶𝗼𝗻 𝗕𝗲𝘁𝘄𝗲𝗲𝗻 𝗟𝗶𝗴𝗵𝘁 & 𝗧𝗵𝗲 𝗕𝗼𝗱𝘆
Light is capable of interacting with biological tissues through various mechanisms including retinal and non-retinal photoreceptors known as opsins, photosensitive compounds such as vitamin D precursors, and porphyrins such as haeme which is present in haemoglobin as well
as mitochondria(3).
Light is capable of interacting with biological tissues through various mechanisms including retinal and non-retinal photoreceptors known as opsins, photosensitive compounds such as vitamin D precursors, and porphyrins such as haeme which is present in haemoglobin as well
as mitochondria(3).
𝗟𝗶𝗴𝗵𝘁 & 𝗧𝗵𝗲 𝗛𝘂𝗺𝗮𝗻 𝗕𝗿𝗮𝗶𝗻
The depth by which light penetrates into the body varies depending on its wavelength.
The depth by which light penetrates into the body varies depending on its wavelength.
UV light mainly affects the skin due to its minimal penetration range, whereas longer wavelengths such as red and near-infrared light can penetrate deep into tissues. 
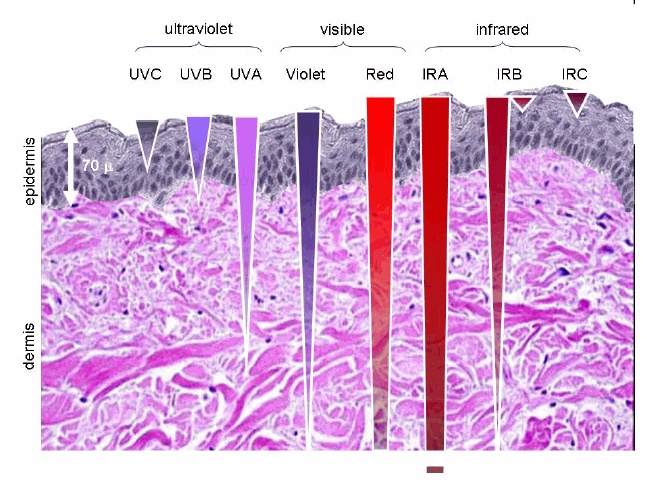
It was shown in rats that light can reach the substantia nigra through the eyes covering a distance of only 80mm, 56mm of which is transparent fluid and only 24mm of tissue(4).
Therefore, light is capable of reaching the substantia nigra and impacting dopaminergic neurons.
Therefore, light is capable of reaching the substantia nigra and impacting dopaminergic neurons.

However, as far as I know, this has not yet been proven in humans.
Interestingly, a direct neural pathway exists projecting from the retina to the substantia nigra via the superior colliculus(5).
Interestingly, a direct neural pathway exists projecting from the retina to the substantia nigra via the superior colliculus(5).
𝗦𝘂𝗻𝗹𝗶𝗴𝗵𝘁 & 𝗣𝗮𝗿𝗸𝗶𝗻𝘀𝗼𝗻’𝘀 𝗗𝗶𝘀𝗲𝗮𝘀𝗲
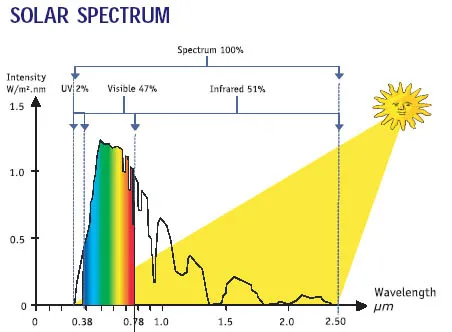
The sun represents the single largest energy input to the human body with a solar spectrum
extending between 250-4000nm.
Near-infrared (NIR) light has wavelengths that range from approximately 800nm to about 2500 nm.
The sun represents the single largest energy input to the human body with a solar spectrum
extending between 250-4000nm.
Near-infrared (NIR) light has wavelengths that range from approximately 800nm to about 2500 nm.
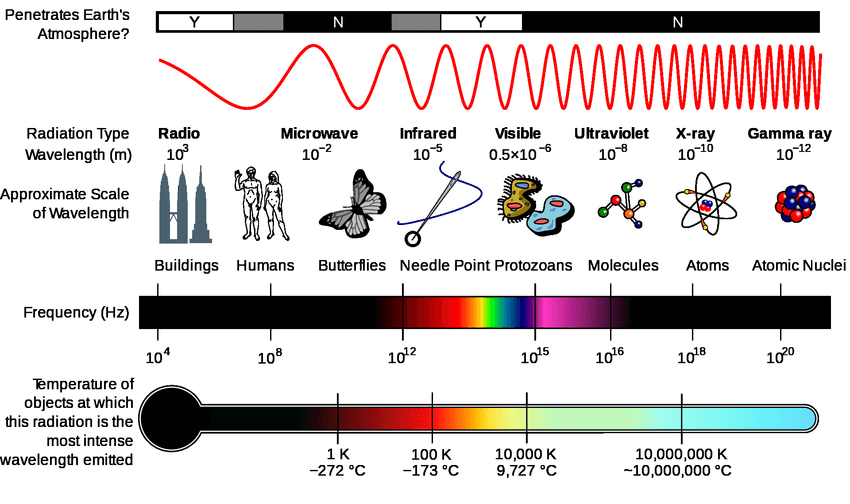
50-70% of the photons emitted from the sun fall in the NIR range.
The human body has evolved over millennia under a near-infrared (NIR) emitter, the Sun (this is crucial to remember).
The human body has evolved over millennia under a near-infrared (NIR) emitter, the Sun (this is crucial to remember).
Epidemiological evidence points towards a relationship between sunlight and Parkinson’s disease.
A geographical relationship between latitude of birth in the US and mortality rates for Parkinson’s disease has been observed(7).
A geographical relationship between latitude of birth in the US and mortality rates for Parkinson’s disease has been observed(7).
Furthermore, a Swedish study found that as latitude increases, so too does the incidence of PD.
More recently, a study of 69,010 participants established a strong negative correlation between vitamin D levels (which are tightly linked to UV-B light exposure) and the incidence of Parkinson’s disease(9).
More recently, a study of 69,010 participants established a strong negative correlation between vitamin D levels (which are tightly linked to UV-B light exposure) and the incidence of Parkinson’s disease(9).

Likewise, circannual variations in the activity of tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis, and dopamine transporter activity have been observed, with elevated levels noted during the summer(10). 
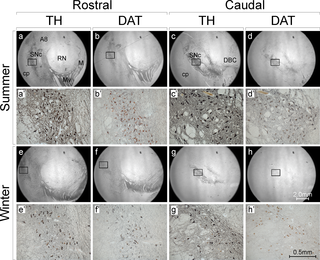
To top is all off, a systematic review found that sunlight exposure was linked with a reduced risk of developing Parkinson’s disease(11). 

𝗟𝗶𝗴𝗵𝘁 𝗧𝗵𝗲𝗿𝗮𝗽𝘆 & 𝗣𝗮𝗿𝗸𝗶𝗻𝘀𝗼𝗻’𝘀 𝗗𝗶𝘀𝗲𝗮𝘀𝗲
Modern applications of light that mimic the electromagnetic spectrum of sunlight have proven beneficial for a multitude of health parameters in circumstances where direct exposure to sunlight is not possible(12).
Modern applications of light that mimic the electromagnetic spectrum of sunlight have proven beneficial for a multitude of health parameters in circumstances where direct exposure to sunlight is not possible(12).

Not only does light treatment prove effective in alleviating insomnia and depression, but it also led to noticeable improvements in motor function for a considerable number of patients with PD(13,14) - this cannot be understated.
Inspired by the success of light therapy in treating depression, including seasonal affective disorder, the study explored whether PD-related depression could also be alleviated through light treatment(15).
This approach was attractive, considering the significant drug burden carried by PD patients.
This approach was attractive, considering the significant drug burden carried by PD patients.
Surprisingly, the preliminary results suggest the use of light therapy not only significantly improved depression but also led to notable reductions in bradykinesia and rigidity, allowing for a substantial reduction in L-dopa by up to 50%(15).
THIS IS HUGE!
THIS IS HUGE!
Dyskinesia was markedly reduced, and patients tolerated 'drug holidays' well, highlighting the potential for non-invasive treatment to simplify regimens and minimise side effects and drug interactions(15).
Motor function typically exhibited gradual improvement, with noticeable effects emerging within 3 to 5 weeks.
Conversely, symptoms of depression, anxiety, and insomnia responded more promptly, often showing improvement within the first week(15).
The authors of this study concluded that “light therapy may be used as monotherapy in patients with initial manifestations of the disease”(15).
The authors of this study concluded that “light therapy may be used as monotherapy in patients with initial manifestations of the disease”(15).
Further studies have confirmed that light can act as an effective adjunct to DART for reducing dyskinesia(16).
A double-blind, placebo controlled trial concluded that light therapy for PD patients 1hr after wakening improves quality of life, mood, as well as associated tremors(17).
A double-blind, placebo controlled trial concluded that light therapy for PD patients 1hr after wakening improves quality of life, mood, as well as associated tremors(17).
It appears that light therapy in close conjunction with strategic doses of DART, if needed, not only attenuates primary motor and non-motor symptoms of PD, but is also capable of steadily improving the disease over months and years(13).
Dopamine and serotonin may well be involved in the therapeutic effect observed after light exposure with both neurotransmitters working to repair locomotion in the same way that they are beneficial in treating depressive symptoms(13). 
It's crucial to highlight that, although bright light therapy offers a brightness of 10,000 lux, natural sunlight surpasses this with levels ranging from 50,000 to 100,000 lux.
The superior therapeutic choice is natural sunlight, primarily because of the balanced electromagnetic spectrum it provides, with a special emphasis on UV-B exposure, which closely correlates with the synthesis of Vitamin D.
The superior therapeutic choice is natural sunlight, primarily because of the balanced electromagnetic spectrum it provides, with a special emphasis on UV-B exposure, which closely correlates with the synthesis of Vitamin D.
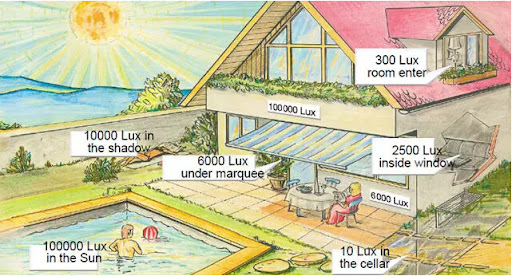
𝗩𝗶𝘁𝗮𝗺𝗶𝗻 𝗗 & 𝗣𝗮𝗿𝗸𝗶𝗻𝘀𝗼𝗻’𝘀 𝗗𝗶𝘀𝗲𝗮𝘀𝗲
UVB (Ultraviolet B) radiation from the sun plays a crucial role in stimulating the synthesis of Vitamin D in the skin.
UVB (Ultraviolet B) radiation from the sun plays a crucial role in stimulating the synthesis of Vitamin D in the skin.
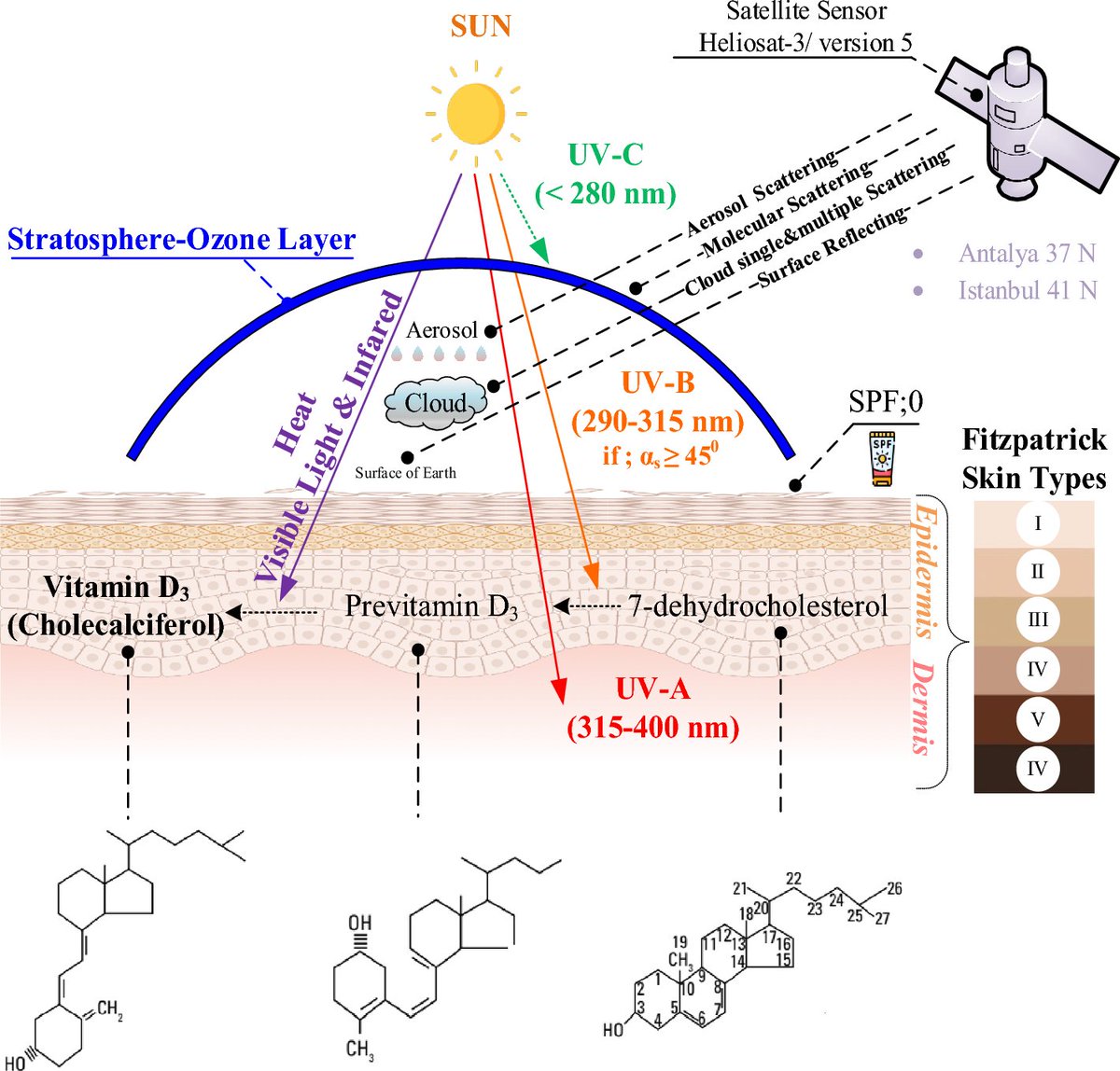
When the skin is exposed to UVB rays, a precursor molecule in the skin, 7-dehydrocholesterol, undergoes a photolytic conversion, transforming into previtamin D3.
This previtamin D3 then undergoes a thermal isomerization process, turning into Vitamin D3 (cholecalciferol).
The liver and kidneys subsequently convert Vitamin D3 into its active form, calcitriol, which plays a vital role in regulating calcium and phosphorus metabolism for bone health and has various other physiological functions in the body. 
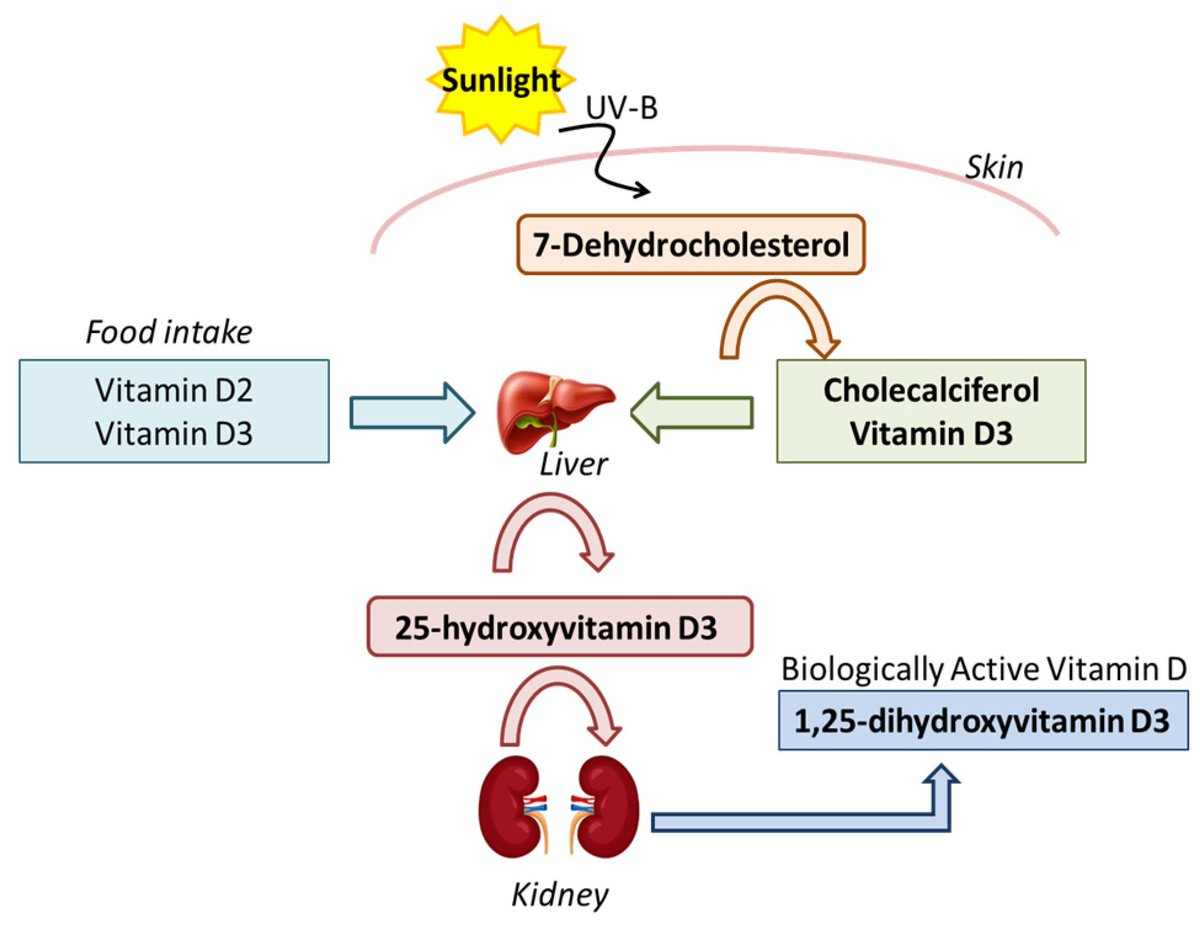
UVB exposure is, therefore, a key factor in maintaining adequate Vitamin D levels in the human body.
A vitamin D deficiency has been shown to be associated with a 300% increase in risk of developing PD(11,18).
A case-control study of 201 patients newly diagnosed with PD and 199 healthy controls found a significant negative correlation between serum vitamin D levels, sunlight exposure and the risk of PD(19).
Vitamin D is essential for regulating key neurodegenerative processes, including nitric oxide synthase function, glutathione and monoamine synthesis(19).
It also plays a role in reducing nervous system oxidative stress, modulating the immune system and facilitating detoxification(20).
Vitamin D also effects gene expression(21).
Among the genes affected by a vitamin D deficiency, the tyrosine hydroxylase (TH) gene is particularly relevant here.
Among the genes affected by a vitamin D deficiency, the tyrosine hydroxylase (TH) gene is particularly relevant here.
This gene, when suppressed due to vitamin D deficiency, is crucial in controlling the biosynthesis of dopamine(22).
Interestingly, high levels of vitamin D receptors and the enzyme responsible for producing the active form 1,25(OH)2D3 has been noted in the substantia nigra.
Ongoing research indicates that persistent vitamin D deficiency could potentially contribute to the degeneration of dopaminergic neurons in the substantia nigra, potentially leading to the onset of PD(23).
Additionally, supplementation with vitamin D3 may temporarily stabilise PD in individuals with certain genotypes(24).
Some studies have shown that vitamin D supplementation can reduce the deterioration of motor function, and animal studies show a reduction in neuroinflammation and dopaminergic neurodegeneration(23,25).
However, a meta-analysis found no symptomatic improvement with vitamin D supplementation, so the scientific literature remains conflicted on this topic(11).
It is important to note that whilst vitamin D is an important by-product of sun exposure, it does not begin to encompass all the photobiological effects of sunlight.
Indeed, adequate sun exposure was associated with 1/50 of the risk of developing PD(11). Another essential element of sunlight is near-infrared light, constituting approximately 50-70% of the photons emitted.
𝗡𝗲𝗮𝗿 𝗜𝗻𝗳𝗿𝗮𝗿𝗲𝗱 𝗟𝗶𝗴𝗵𝘁 & 𝗣𝗮𝗿𝗸𝗶𝗻𝘀𝗼𝗻’𝘀 𝗗𝗶𝘀𝗲𝗮𝘀𝗲
The strategic use of photobiomodulation has been shown to provide therapeutic benefits in PD.
The strategic use of photobiomodulation has been shown to provide therapeutic benefits in PD.
Applying near-infrared light locally to the substantia nigra demonstrates neuroprotective effects in Parkinson's disease models.(3). 
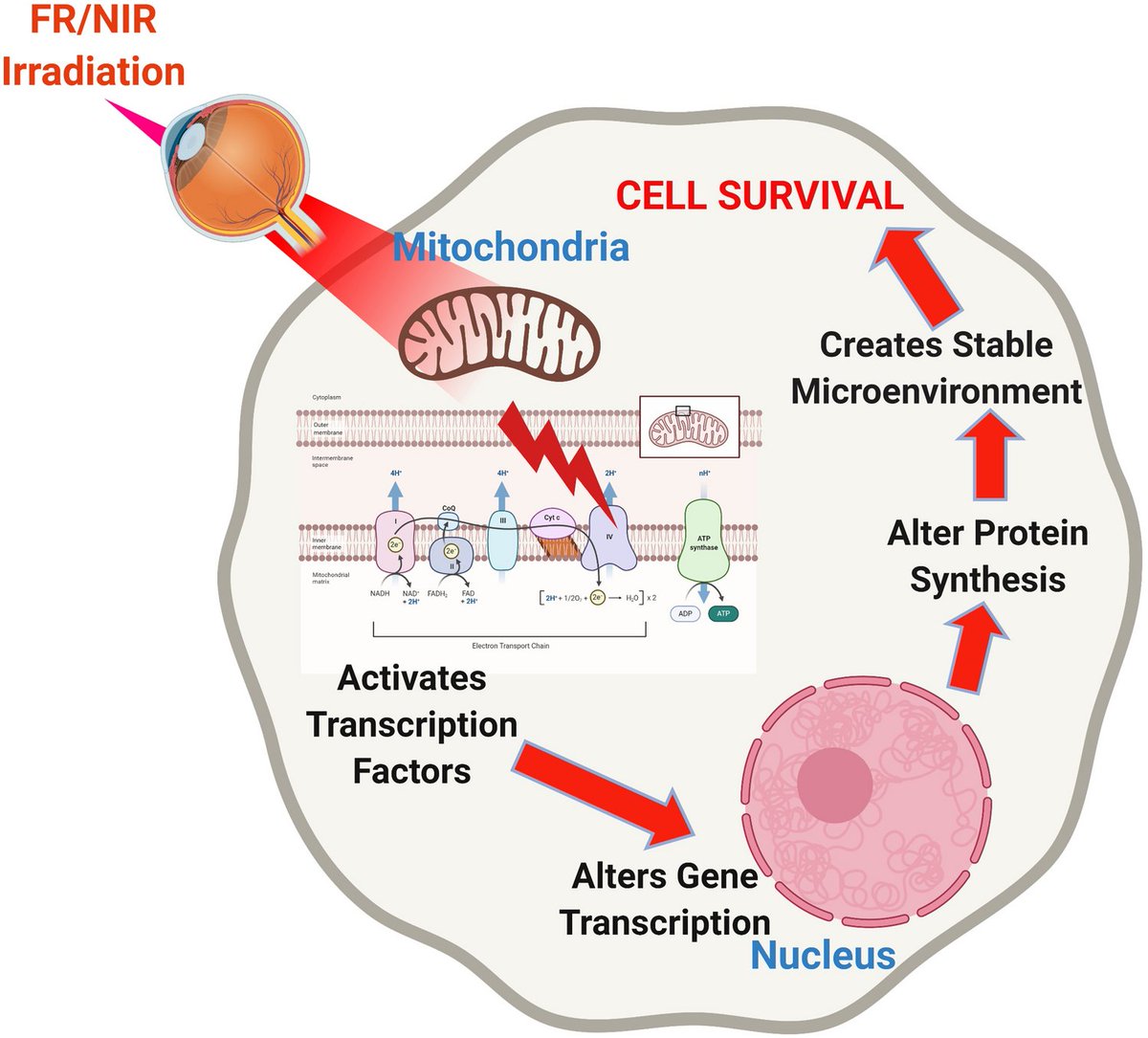
In animal studies, near-infrared light has been observed to play both a neuroprotective and rescuing role in response to the neurotoxic
agent MPTP(26,27).
agent MPTP(26,27).
Several mechanisms may explain the beneficial effects of near-infrared light in PD.
Cytochrome c oxidase, a photoacceptor present in the electron transport chain of mitochondria, absorbs near-infrared light, enhancing its electron transfer capabilities and ATP production(28).
Cytochrome c oxidase, a photoacceptor present in the electron transport chain of mitochondria, absorbs near-infrared light, enhancing its electron transfer capabilities and ATP production(28).
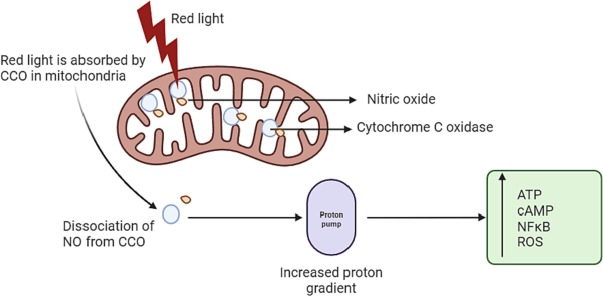
Additionally, the dissociation of nitric oxide from mitochondria triggered by near-infrared light induces vasodilation, leading to enhanced blood flow.
There are likely effects regarding water structure and protein folding as well.
There are likely effects regarding water structure and protein folding as well.
This, in turn, improves the delivery of oxygen and glucose to neurons and facilitates the removal of toxins(28).
Indeed, PD has been associated with heavy metal toxicity as well as pesticide induced neuroinflammation(29,30).
Indeed, PD has been associated with heavy metal toxicity as well as pesticide induced neuroinflammation(29,30).
Furthermore, near-infrared light induces a short burst of reactive oxygen species, elevating the expression of protective genes such as GDNF.
This results in enduring benefits that can last for weeks or even months following exposure(28).
This results in enduring benefits that can last for weeks or even months following exposure(28).
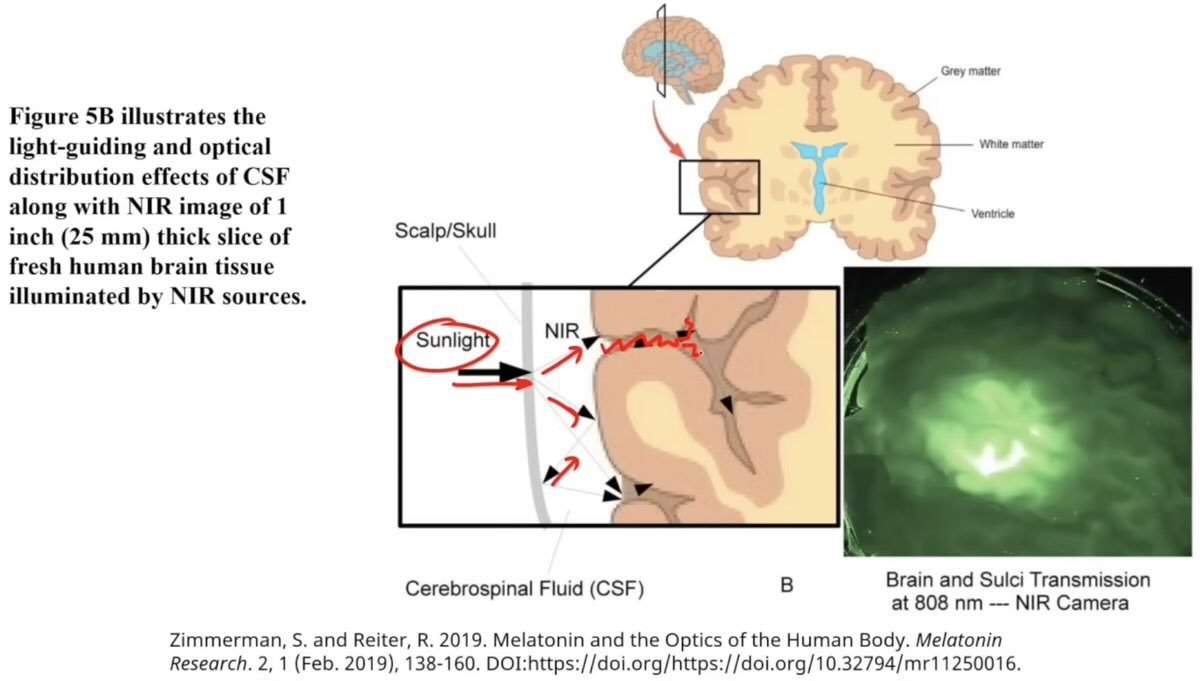
Interestingly, while the skull blocks UV photons, it effectively transmits near-infrared photons.
The cerebral spinal fluid surrounding the brain also has its minimum optical absorption spectrum in the near-infrared range allowing it to permeate through the fluid and scatter on surrounding surfaces(6).
The cerebral spinal fluid surrounding the brain also has its minimum optical absorption spectrum in the near-infrared range allowing it to permeate through the fluid and scatter on surrounding surfaces(6).
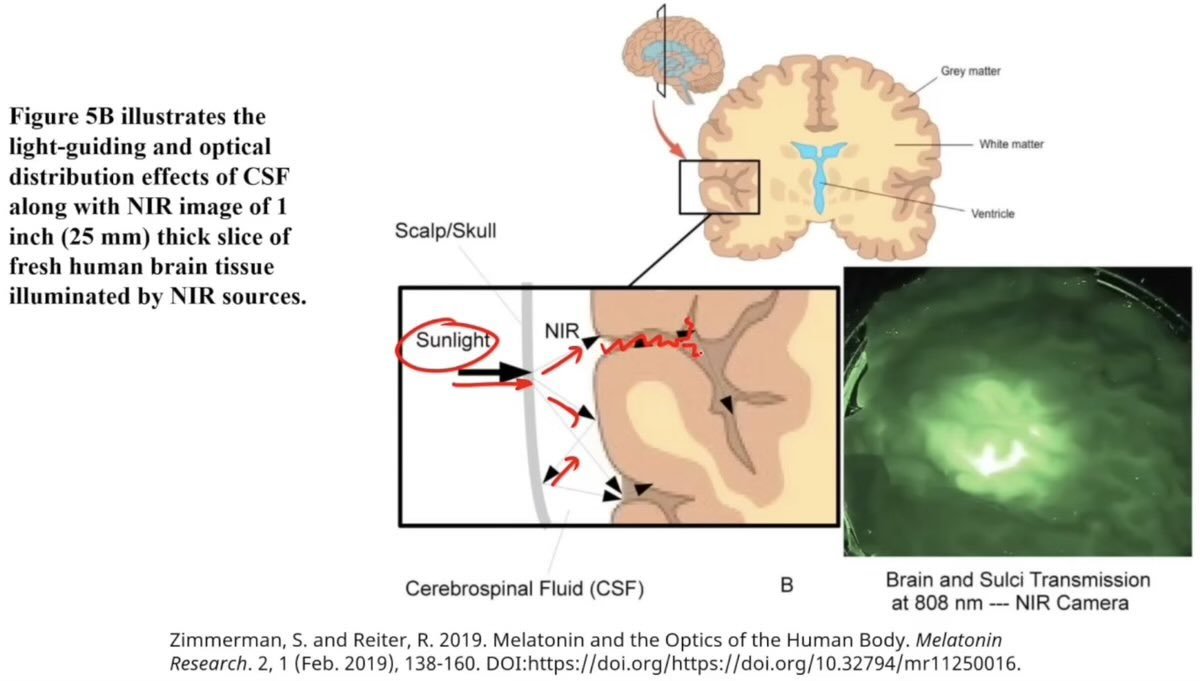
The brain's optics are designed in such a way as to distribute near-infrared photons to the grey matter, including even the deepest recesses of the brain(6).
It is no coincidence that the grey matter is located on the outside surface of the brain, and that the blood supply to the brain organizes itself starting from the outside and moving inward.
Neuromelanin in the gray matter has a very strong capacity to absorb near-infrared light compared to the underlying white matter(6).
The substantia nigra which is implicated in PD gains its dark appearance through concentrating neuromelanin.
Unfortunately, our light environment is polluted by artificial light sources. LED & fluorescent lighting are "visible only" sources and emit no NIR photons.
Unfortunately, our light environment is polluted by artificial light sources. LED & fluorescent lighting are "visible only" sources and emit no NIR photons.
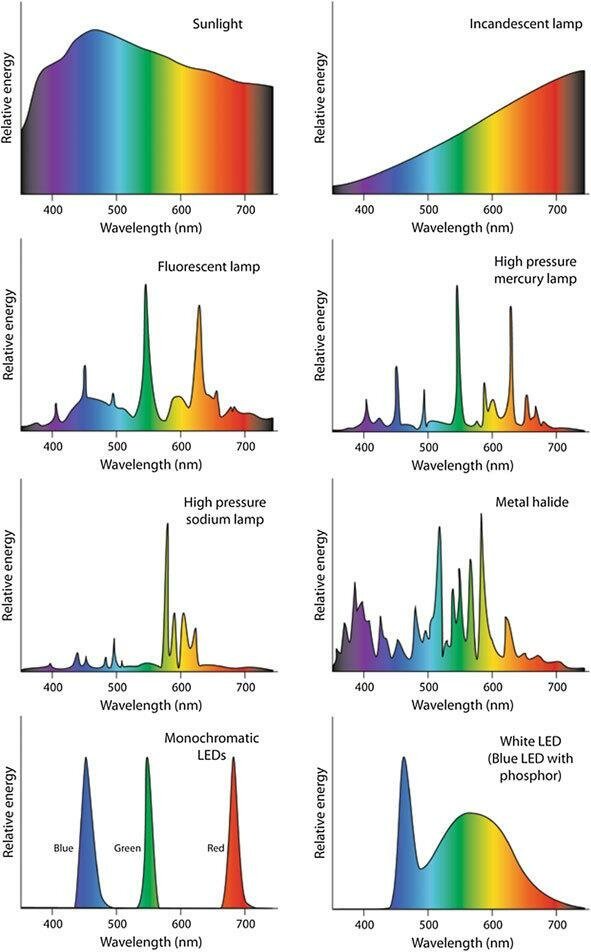
𝗔𝗿𝘁𝗶𝗳𝗶𝗰𝗶𝗮𝗹 𝗟𝗶𝗴𝗵𝘁 & 𝗣𝗮𝗿𝗸𝗶𝗻𝘀𝗼𝗻’𝘀 𝗗𝗶𝘀𝗲𝗮𝘀𝗲
Excessive exposure to artificial fluorescent light can disrupt circadian rhythms, impair sleep and harm dopaminergic neurons.
This could be a potential risk factor for Parkinson’s Disease(3).
Excessive exposure to artificial fluorescent light can disrupt circadian rhythms, impair sleep and harm dopaminergic neurons.
This could be a potential risk factor for Parkinson’s Disease(3).
Though it does not signify a causal link, there exists a significant correlation between locational light pollution and the prevalence of PD(31).
Additionally, individuals with higher levels of education exhibit a greater prevalence of Parkinson's disease, potentially attributed to increased exposure to artificial light sources, such as screens(32).
Computer programmers are particularly vulnerable to developing Parkinson’s disease(33).
Animal studies have shown a 30% reduction in the quantity of dopaminergic neurons in the substantia nigra when exposed to continuous artificial light for 3 months(31,34).
Animal studies have shown a 30% reduction in the quantity of dopaminergic neurons in the substantia nigra when exposed to continuous artificial light for 3 months(31,34).
It appears that optimising exposure to natural sunlight, whilst minimising the effects of artificial light sources may significantly improve the quality of life of those with PD.
𝗟𝗶𝗴𝗵𝘁, 𝗣𝗮𝗶𝗻 & 𝗣𝗮𝗿𝗸𝗶𝗻𝘀𝗼𝗻’𝘀 𝗗𝗶𝘀𝗲𝗮𝘀𝗲
Another mechanism by which sunlight may contribute to the overall well-being of those with PD is the production of beta-endorphins upon exposure to UV-B radiation(35).
Another mechanism by which sunlight may contribute to the overall well-being of those with PD is the production of beta-endorphins upon exposure to UV-B radiation(35).
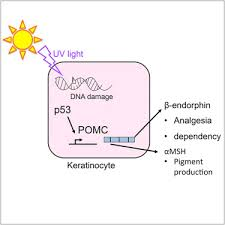
These opioid peptides have the effect of enhancing mood, strengthening the immune system, alleviating pain, inducing relaxation, aiding in the healing of wounds, and supporting cellular differentiation.
Given that pain is a prevalent symptom in PD, chronic pain being twice as prevalent in those with Parkinson's compared to those without, understanding that sunlight exposure can provide relief becomes crucial(36).
𝗖𝗶𝗿𝗰𝗮𝗱𝗶𝗮𝗻 𝗥𝗵𝘆𝘁𝗵𝗺𝘀 & 𝗣𝗮𝗿𝗸𝗶𝗻𝘀𝗼𝗻’𝘀 𝗗𝗶𝘀𝗲𝗮𝘀𝗲
Sleep disturbances and disorders, encompassing reduced total sleep time, decreased sleep efficiency, heightened sleep fragmentation, REM sleep behaviour disorder, and excessive daytime sleepiness, are observed in approximately 60–95% of PD patients(37).
Sleep disturbances and disorders, encompassing reduced total sleep time, decreased sleep efficiency, heightened sleep fragmentation, REM sleep behaviour disorder, and excessive daytime sleepiness, are observed in approximately 60–95% of PD patients(37).
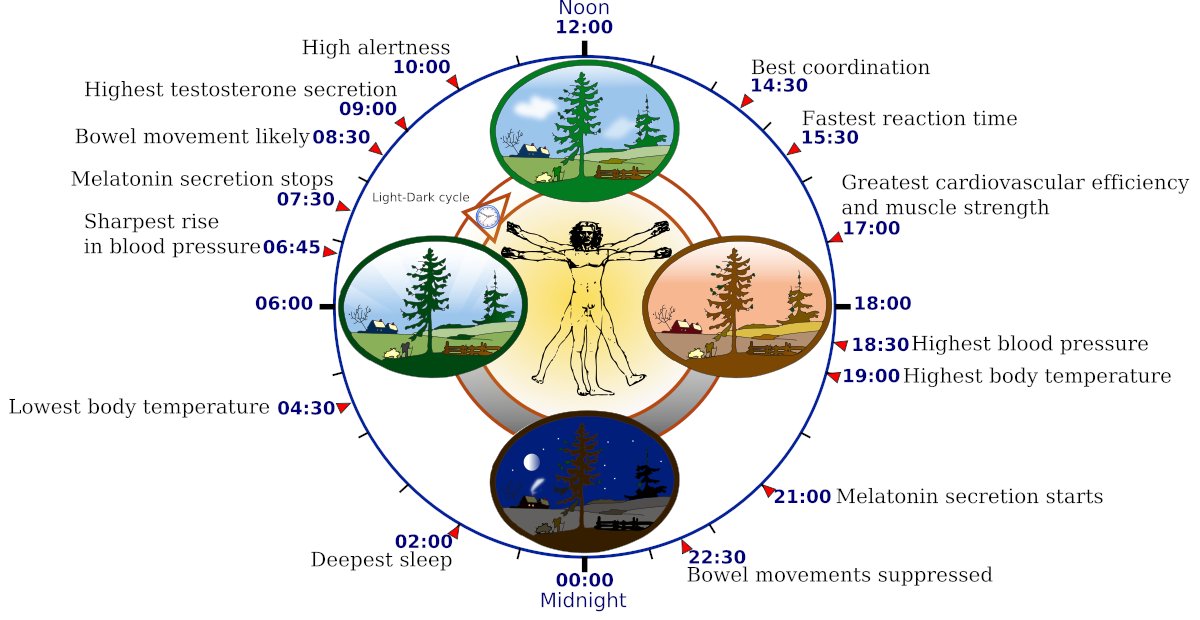
Disrupted sleep is reported in 64.1% of individuals with non-motor symptoms of Parkinson's disease(38). Indeed, abnormal dopamine function may be related to impaired sleep.
Furthermore, depression, insomnia, and nocturnal myoclonus, all recognized to be regulated by circadian rhythms, are evident in individuals with Parkinson's disease(13).
This highlights the importance of circadian health when approaching the management of PD from a holistic perspective.
This highlights the importance of circadian health when approaching the management of PD from a holistic perspective.
Circadian rhythms are endogenous biological oscillations that regulate numerous physiological processes, including sleep-wake cycles, hormone secretion, body temperature, metabolism, digestion as well as cellular maintenance and repair.
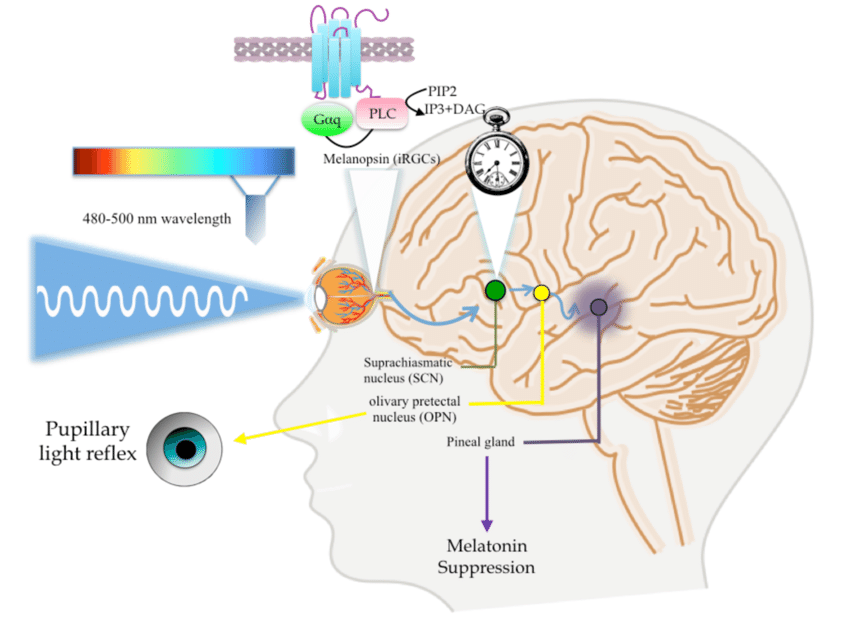
The circadian rhythm is entrained by environmental cues acting on sensors within the retina containing melanopsin.
Melanopsin is a photopigment and chromophore found in the intrinsically photosensitive retinal ganglion cells (ipRGCs) that is sensitive to blue light.
Melanopsin is a photopigment and chromophore found in the intrinsically photosensitive retinal ganglion cells (ipRGCs) that is sensitive to blue light.
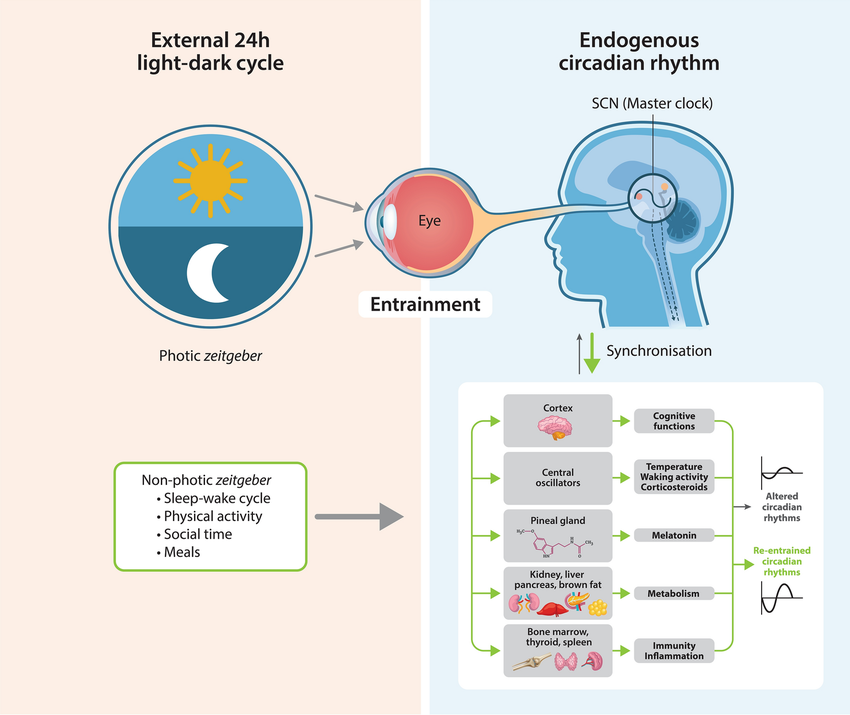
Upon exposure to blue light, melanopsin coordinates with the suprachiasmatic nucleus (SCN) located in the hypothalamus to synchronise the body's circadian rhythms with the light and dark cycle. 
The human body relies on natural light exposure, particularly in the morning, to regulate its internal circadian clocks effectively.
Exposure to natural light synchronises endogenous rhythms and behaviour via circadian entrainment of the endogenous pacemaker within the suprachiasmatic nucleus.
Exposure to natural light synchronises endogenous rhythms and behaviour via circadian entrainment of the endogenous pacemaker within the suprachiasmatic nucleus.
It has been shown that natural sunlight is crucial to entraining circadian rhythms, and is in fact more important for circadian health than avoiding artificial light at night.
The neurodegenerative process in PD, resulting in dopamine depletion, is considered one of the underlying causes of circadian disruption.
A direct link between dopamine and the circadian rhythm has been established(37).
A direct link between dopamine and the circadian rhythm has been established(37).
Clock proteins like PER2 appear to regulate striatal dopamine metabolism(39).
Conversely, dopamine receptor stimulation influences the rhythmic expression of clock genes such as PER1 and PER2 in the striatum(40).
Conversely, dopamine receptor stimulation influences the rhythmic expression of clock genes such as PER1 and PER2 in the striatum(40).
Additionally, dopamine regulates the rhythmic expression of melanopsin in retinal ganglion cells, influencing the entrainment of the circadian rhythm by light(41).
The relationship between circadian rhythm disruption and PD may be bidirectional.
The relationship between circadian rhythm disruption and PD may be bidirectional.
Circadian rhythm disruption has been established as a risk factor for PD by triggering neuroinflammation and subsequent degeneration of dopaminergic neurons within the substantia nigra(42).
In numerous studies, light therapy has been applied in the treatment of excessive daytime sleepiness in PD.
For instance, a randomised clinical trial revealed that 14 days of bright light therapy not only improved excessive daytime sleepiness, sleep fragmentation, and sleep quality but also led to better sleep latency and overall sleep quality, with sustained benefits even after a 2-week "washout" period(43,44).
These therapeutic effects are likely attributed to the synchronisation of the circadian clock and the restoration of circadian rhythms(3).
Importantly, patients also experienced a reduction in the severity of their motor disturbances.
Importantly, patients also experienced a reduction in the severity of their motor disturbances.
Similar improvements in motor symptoms were observed in other studies suggesting that light therapy could serve as a valuable complement or even alternative to pharmacological treatment in Parkinson's disease(13,16).
That's the end! 🦁
Thanks for reading, please consider liking & retweeting the first tweet (linked below) to share it around because this information can help a lot of people dealing with PD!
Thanks for reading, please consider liking & retweeting the first tweet (linked below) to share it around because this information can help a lot of people dealing with PD!
https://x.com/sam_soete/status/1948367685114269941
𝗥𝗲𝗳𝗲𝗿𝗲𝗻𝗰𝗲𝘀
1. Ceravolo R, Frosini D, Rossi C, Bonuccelli U. Spectrum of addictions in Parkinson’s
disease: from dopamine dysregulation syndrome to impulse control disorders. J Neurol.
2010 Nov;257(Suppl 2):S276-283.
2. Hinson VK. Parkinson’s disease and motor fluctuations. Curr Treat Options Neurol. 2010
May;12(3):186–99.
3. Maggio R, Vaglini F, Rossi M, Fasciani I, Pietrantoni I, Marampon F, et al. Parkinson’s
disease and light: The bright and the Dark sides. Brain Research Bulletin. 2019 Aug
1;150:290–6.
4. Romeo S, Di Camillo D, Splendiani A, Capannolo M, Rocchi C, Aloisi G, et al. Eyes as
Gateways for Environmental Light to the Substantia Nigra: Relevance in Parkinson’s
Disease. ScientificWorldJournal. 2014 Jan 22;2014:317879.
5. Dommett E, Coizet V, Blaha CD, Martindale J, Lefebvre V, Walton N, et al. How Visual
Stimuli Activate Dopaminergic Neurons at Short Latency. Science. 2005 Mar
4;307(5714):1476–9.
6. Zimmerman S, Reiter RJ. Melatonin and the Optics of the Human Body. Melatonin
Research. 2019 Feb 28;2(1):138–60.
7. Kurtzke JF, Goldberg ID. Parkinsonism death rates by race, sex, and geography.
Neurology. 1988;38(10):1558–61.
8. de Pedro Cuesta J. Studies on the prevalence of paralysis agitans by tracer methodology. Acta Neurol Scand Suppl. 1987;112:1–106.
9. Evatt ML, DeLong MR, Khazai N, Rosen A, Triche S, Tangpricha V. Prevalence of
Vitamin D Insufficiency in Patients With Parkinson Disease and Alzheimer Disease.
Archives of Neurology. 2008 Oct 1;65(10):1348–52.
10. Aumann TD, Raabus M, Tomas D, Prijanto A, Churilov L, Spitzer NC, et al. Differences
in Number of Midbrain Dopamine Neurons Associated with Summer and Winter
Photoperiods in Humans. PLOS ONE. 2016 Jul 18;11(7):e0158847.
11. Zhou Z, Zhou R, Zhang Z, Li K. The Association Between Vitamin D Status, Vitamin D
Supplementation, Sunlight Exposure, and Parkinson’s Disease: A Systematic Review
and Meta-Analysis. Med Sci Monit. 2019 Jan 23;25:666–74.
12. West A, Jennum P, Simonsen SA, Sander B, Pavlova M, Iversen HK. Impact of
naturalistic lighting on hospitalized stroke patients in a rehabilitation unit: Design and
measurement. Chronobiology International. 2017 Jul 3;34(6):687–97.
13. Willis GL, Moore C, Armstrong SM. A historical justification for and retrospective analysis of the systematic application of light therapy in Parkinson’s disease. Rev Neurosci. 2012 Mar 1;23(2):199–226.
14. Willis GL, Armstrong SM. A therapeutic role for melatonin antagonism in experimental
models of Parkinson’s disease. Physiol Behav. 1999 Jul;66(5):785–95.
15. Artemenko AR, Levin II. [The phototherapy of parkinsonism patients]. Zh Nevrol Psikhiatr Im S S Korsakova. 1996;96(3):63–6.
16. Willis GL, Turner EJD. Primary and Secondary Features of Parkinson’s Disease Improve with Strategic Exposure to Bright Light: A Case Series Study. Chronobiology International. 2007 Jan 1;24(3):521–37.
17. Paus S, Schmitz-Hübsch T, Wüllner U, Vogel A, Klockgether T, Abele M. Bright light
therapy in Parkinson’s disease: a pilot study. Mov Disord. 2007 Jul 30;22(10):1495–8.
9
18. McSharry C. Could sunlight offer protection from Parkinson disease? Nat Rev Neurol.
2010 Sep;6(9):468–468.
19. Wang J, Yang D, Yu Y, Shao G, Wang Q. Vitamin D and Sunlight Exposure in
Newly-Diagnosed Parkinson’s Disease. Nutrients. 2016 Mar 4;8(3):142.
20. Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing
“D”ecline? Mol Aspects Med. 2008 Dec;29(6):415–22.
21. Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, et al.
Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin
D3 target genes. Mol Endocrinol. 2005 Nov;19(11):2685–95.
22. Puchacz E, Stumpf WE, Stachowiak EK, Stachowiak MK. Vitamin D increases
expression of the tyrosine hydroxylase gene in adrenal medullary cells. Brain Res Mol
Brain Res. 1996 Feb;36(1):193–6.
23. Suzuki M, Yoshioka M, Hashimoto M, Murakami M, Noya M, Takahashi D, et al.
Randomized, double-blind, placebo-controlled trial of vitamin D supplementation in
Parkinson disease. Am J Clin Nutr. 2013 May;97(5):1004–13.
24. Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Sääksjärvi K, Heliövaara M. Serum
vitamin D and the risk of Parkinson disease. Arch Neurol. 2010 Jul;67(7):808–11.
25. Calvello R, Cianciulli A, Nicolardi G, De Nuccio F, Giannotti L, Salvatore R, et al. Vitamin
D Treatment Attenuates Neuroinflammation and Dopaminergic Neurodegeneration in an
Animal Model of Parkinson’s Disease, Shifting M1 to M2 Microglia Responses. J Neuroimmune Pharmacol. 2017 Jun;12(2):327–39.
26. Shaw VE, Spana S, Ashkan K, Benabid AL, Stone J, Baker GE, et al. Neuroprotection of
midbrain dopaminergic cells in MPTP-treated mice after near-infrared light treatment.
Journal of Comparative Neurology. 2010;518(1):25–40.
27. Darlot F, Moro C, El Massri N, Chabrol C, Johnstone DM, Reinhart F, et al. Near-infrared
light is neuroprotective in a monkey model of Parkinson disease. Annals of Neurology.
2016;79(1):59–75.
28. Mitrofanis J. Why and how does light therapy offer neuroprotection in Parkinson’s
disease? Neural Regeneration Research. 2017 Apr;12(4):574.
29. Vellingiri B, Suriyanarayanan A, Abraham KS, Venkatesan D, Iyer M, Raj N, et al.
Influence of heavy metals in Parkinson’s disease: an overview. J Neurol. 2022 Nov
1;269(11):5798–811.
30. Bloem BR, Boonstra TA. The inadequacy of current pesticide regulations for protecting
brain health: the case of glyphosate and Parkinson’s disease. The Lancet Planetary
Health. 2023 Dec 1;7(12):e948–9.
31. Romeo S, Viaggi C, Di Camillo D, Willis AW, Lozzi L, Rocchi C, et al. Bright light
exposure reduces TH-positive dopamine neurons: implications of light pollution in
Parkinson’s disease epidemiology. Sci Rep. 2013 Mar 6;3:1395.
32. Frigerio R, Elbaz A, Sanft KR, Peterson BJ, Bower JH, Ahlskog JE, et al. Education and
occupations preceding Parkinson disease. Neurology. 2005 Nov 22;65(10):1575–83.
10
33. Goldman SM, Tanner CM, Olanow CW, Watts RL, Field RD, Langston JW. Occupation
and parkinsonism in three movement disorders clinics. Neurology. 2005 Nov
8;65(9):1430–5.
34. Taufique SKT, Kumar V. Differential activation and tyrosine hydroxylase distribution in the
hippocampal, pallial and midbrain brain regions in response to cognitive performance in
Indian house crows exposed to abrupt light environment. Behavioural Brain Research.
2016 Nov 1;314:21–9.
35. Fell GL, Robinson KC, Mao J, Woolf CJ, Fisher DE. Skin β-endorphin mediates
addiction to ultraviolet light. Cell. 2014 Jun 19;157(7):1527–34.
36. Mylius V, Perez Lloret S, Cury RG, Teixeira MJ, Barbosa VR, Barbosa ER, et al. The
Parkinson disease pain classification system: results from an international
mechanism-based classification approach. PAIN. 2021 Apr;162(4):1201.
37. Rutten S, Vriend C, van den Heuvel OA, Smit JH, Berendse HW, van der Werf YD.
Bright Light Therapy in Parkinson’s Disease: An Overview of the Background and
Evidence. Parkinson’s Disease. 2012 Dec 23;2012:e767105.
38. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact
on quality of life in Parkinson’s disease - Barone - 2009 - Movement Disorders.
39. Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, et al.
Regulation of Monoamine Oxidase A by Circadian-Clock Components Implies Clock
Influence on Mood. Current Biology. 2008 May 6;18(9):678–83.
40. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in
the rat dorsal striatum via daily activation of D2 dopamine receptors.
41. Sakamoto K, Liu C, Kasamatsu M, Pozdeyev NV, Iuvone PM, Tosini G. Dopamine
regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion
cells. Eur J Neurosci. 2005 Dec;22(12):3129–36.
42. Lauretti E, Di Meco A, Merali S, Praticò D. Circadian rhythm dysfunction: a novel
environmental risk factor for Parkinson’s disease. Mol Psychiatry. 2017 Feb;22(2):280–6.
43. Videnovic A, Klerman EB, Wang W, Marconi A, Kuhta T, Zee PC. Timed Light Therapy
for Sleep and Daytime Sleepiness Associated With Parkinson Disease: A Randomized
Clinical Trial. JAMA Neurology. 2017 Apr 1;74(4):411–8.
44. Fifel K, Videnovic A. Chronotherapies for Parkinson’s disease. Progress in Neurobiology.
2019 Mar 1;174:16–27.
45. College of Optometry [Internet]. 2016 [cited 2023 Dec 28]. Scientists study effects of
sunlight to reduce number of nearsighted kids.
46. Isaac W. A study of the relationship between the visual system and the effects of
d-amphetamine. Physiology & Behavior. 1971 Feb 1;6(2):157–9.
47. Kallman WM, Isaac W. The effects of age and illumination on the dose-response curves
for three stimulants. Psychopharmacologia. 1975 Dec 1;40(4):313–8.
48. Pum ME, Rubio AR, Carey RJ, Silva MADS, Müller CP. The effects of cocaine on
light-induced activity. Brain Res Bull. 2011 Feb 28;84(3):229–34.
49. Willis GL. Intraocular microinjections repair experimental Parkinson’s disease. Brain
Research. 2008 Jun 27;1217:119–31.
50. Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, et al. Circadian Melatonin
Rhythm and Excessive Daytime Sleepiness in Parkinson Disease. JAMA Neurology.
2014 Apr 1;71(4):463–9.
51. Naskar A, Prabhakar V, Singh R, Dutta D, Mohanakumar KP. Melatonin enhances
L-DOPA therapeutic effects, helps to reduce its dose, and protects dopaminergic
neurons in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice.
Journal of Pineal Research. 2015;58(3):262–74.
52. Sun J, Lin XM, Lu DH, Wang M, Li K, Li SR, et al. Midbrain dopamine oxidation links
ubiquitination of glutathione peroxidase 4 to ferroptosis of dopaminergic neurons.
53. Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain
dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and
neurochemical correlations. J Neurol Sci. 1973 Dec;20(4):415–55.
54. Willis GL. Parkinson’s disease as a neuroendocrine disorder of circadian function:
dopamine-melatonin imbalance and the visual system in the genesis and progression of
the degenerative process. Rev Neurosci. 2008;19(4–5):245–316.
1. Ceravolo R, Frosini D, Rossi C, Bonuccelli U. Spectrum of addictions in Parkinson’s
disease: from dopamine dysregulation syndrome to impulse control disorders. J Neurol.
2010 Nov;257(Suppl 2):S276-283.
2. Hinson VK. Parkinson’s disease and motor fluctuations. Curr Treat Options Neurol. 2010
May;12(3):186–99.
3. Maggio R, Vaglini F, Rossi M, Fasciani I, Pietrantoni I, Marampon F, et al. Parkinson’s
disease and light: The bright and the Dark sides. Brain Research Bulletin. 2019 Aug
1;150:290–6.
4. Romeo S, Di Camillo D, Splendiani A, Capannolo M, Rocchi C, Aloisi G, et al. Eyes as
Gateways for Environmental Light to the Substantia Nigra: Relevance in Parkinson’s
Disease. ScientificWorldJournal. 2014 Jan 22;2014:317879.
5. Dommett E, Coizet V, Blaha CD, Martindale J, Lefebvre V, Walton N, et al. How Visual
Stimuli Activate Dopaminergic Neurons at Short Latency. Science. 2005 Mar
4;307(5714):1476–9.
6. Zimmerman S, Reiter RJ. Melatonin and the Optics of the Human Body. Melatonin
Research. 2019 Feb 28;2(1):138–60.
7. Kurtzke JF, Goldberg ID. Parkinsonism death rates by race, sex, and geography.
Neurology. 1988;38(10):1558–61.
8. de Pedro Cuesta J. Studies on the prevalence of paralysis agitans by tracer methodology. Acta Neurol Scand Suppl. 1987;112:1–106.
9. Evatt ML, DeLong MR, Khazai N, Rosen A, Triche S, Tangpricha V. Prevalence of
Vitamin D Insufficiency in Patients With Parkinson Disease and Alzheimer Disease.
Archives of Neurology. 2008 Oct 1;65(10):1348–52.
10. Aumann TD, Raabus M, Tomas D, Prijanto A, Churilov L, Spitzer NC, et al. Differences
in Number of Midbrain Dopamine Neurons Associated with Summer and Winter
Photoperiods in Humans. PLOS ONE. 2016 Jul 18;11(7):e0158847.
11. Zhou Z, Zhou R, Zhang Z, Li K. The Association Between Vitamin D Status, Vitamin D
Supplementation, Sunlight Exposure, and Parkinson’s Disease: A Systematic Review
and Meta-Analysis. Med Sci Monit. 2019 Jan 23;25:666–74.
12. West A, Jennum P, Simonsen SA, Sander B, Pavlova M, Iversen HK. Impact of
naturalistic lighting on hospitalized stroke patients in a rehabilitation unit: Design and
measurement. Chronobiology International. 2017 Jul 3;34(6):687–97.
13. Willis GL, Moore C, Armstrong SM. A historical justification for and retrospective analysis of the systematic application of light therapy in Parkinson’s disease. Rev Neurosci. 2012 Mar 1;23(2):199–226.
14. Willis GL, Armstrong SM. A therapeutic role for melatonin antagonism in experimental
models of Parkinson’s disease. Physiol Behav. 1999 Jul;66(5):785–95.
15. Artemenko AR, Levin II. [The phototherapy of parkinsonism patients]. Zh Nevrol Psikhiatr Im S S Korsakova. 1996;96(3):63–6.
16. Willis GL, Turner EJD. Primary and Secondary Features of Parkinson’s Disease Improve with Strategic Exposure to Bright Light: A Case Series Study. Chronobiology International. 2007 Jan 1;24(3):521–37.
17. Paus S, Schmitz-Hübsch T, Wüllner U, Vogel A, Klockgether T, Abele M. Bright light
therapy in Parkinson’s disease: a pilot study. Mov Disord. 2007 Jul 30;22(10):1495–8.
9
18. McSharry C. Could sunlight offer protection from Parkinson disease? Nat Rev Neurol.
2010 Sep;6(9):468–468.
19. Wang J, Yang D, Yu Y, Shao G, Wang Q. Vitamin D and Sunlight Exposure in
Newly-Diagnosed Parkinson’s Disease. Nutrients. 2016 Mar 4;8(3):142.
20. Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing
“D”ecline? Mol Aspects Med. 2008 Dec;29(6):415–22.
21. Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, et al.
Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin
D3 target genes. Mol Endocrinol. 2005 Nov;19(11):2685–95.
22. Puchacz E, Stumpf WE, Stachowiak EK, Stachowiak MK. Vitamin D increases
expression of the tyrosine hydroxylase gene in adrenal medullary cells. Brain Res Mol
Brain Res. 1996 Feb;36(1):193–6.
23. Suzuki M, Yoshioka M, Hashimoto M, Murakami M, Noya M, Takahashi D, et al.
Randomized, double-blind, placebo-controlled trial of vitamin D supplementation in
Parkinson disease. Am J Clin Nutr. 2013 May;97(5):1004–13.
24. Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Sääksjärvi K, Heliövaara M. Serum
vitamin D and the risk of Parkinson disease. Arch Neurol. 2010 Jul;67(7):808–11.
25. Calvello R, Cianciulli A, Nicolardi G, De Nuccio F, Giannotti L, Salvatore R, et al. Vitamin
D Treatment Attenuates Neuroinflammation and Dopaminergic Neurodegeneration in an
Animal Model of Parkinson’s Disease, Shifting M1 to M2 Microglia Responses. J Neuroimmune Pharmacol. 2017 Jun;12(2):327–39.
26. Shaw VE, Spana S, Ashkan K, Benabid AL, Stone J, Baker GE, et al. Neuroprotection of
midbrain dopaminergic cells in MPTP-treated mice after near-infrared light treatment.
Journal of Comparative Neurology. 2010;518(1):25–40.
27. Darlot F, Moro C, El Massri N, Chabrol C, Johnstone DM, Reinhart F, et al. Near-infrared
light is neuroprotective in a monkey model of Parkinson disease. Annals of Neurology.
2016;79(1):59–75.
28. Mitrofanis J. Why and how does light therapy offer neuroprotection in Parkinson’s
disease? Neural Regeneration Research. 2017 Apr;12(4):574.
29. Vellingiri B, Suriyanarayanan A, Abraham KS, Venkatesan D, Iyer M, Raj N, et al.
Influence of heavy metals in Parkinson’s disease: an overview. J Neurol. 2022 Nov
1;269(11):5798–811.
30. Bloem BR, Boonstra TA. The inadequacy of current pesticide regulations for protecting
brain health: the case of glyphosate and Parkinson’s disease. The Lancet Planetary
Health. 2023 Dec 1;7(12):e948–9.
31. Romeo S, Viaggi C, Di Camillo D, Willis AW, Lozzi L, Rocchi C, et al. Bright light
exposure reduces TH-positive dopamine neurons: implications of light pollution in
Parkinson’s disease epidemiology. Sci Rep. 2013 Mar 6;3:1395.
32. Frigerio R, Elbaz A, Sanft KR, Peterson BJ, Bower JH, Ahlskog JE, et al. Education and
occupations preceding Parkinson disease. Neurology. 2005 Nov 22;65(10):1575–83.
10
33. Goldman SM, Tanner CM, Olanow CW, Watts RL, Field RD, Langston JW. Occupation
and parkinsonism in three movement disorders clinics. Neurology. 2005 Nov
8;65(9):1430–5.
34. Taufique SKT, Kumar V. Differential activation and tyrosine hydroxylase distribution in the
hippocampal, pallial and midbrain brain regions in response to cognitive performance in
Indian house crows exposed to abrupt light environment. Behavioural Brain Research.
2016 Nov 1;314:21–9.
35. Fell GL, Robinson KC, Mao J, Woolf CJ, Fisher DE. Skin β-endorphin mediates
addiction to ultraviolet light. Cell. 2014 Jun 19;157(7):1527–34.
36. Mylius V, Perez Lloret S, Cury RG, Teixeira MJ, Barbosa VR, Barbosa ER, et al. The
Parkinson disease pain classification system: results from an international
mechanism-based classification approach. PAIN. 2021 Apr;162(4):1201.
37. Rutten S, Vriend C, van den Heuvel OA, Smit JH, Berendse HW, van der Werf YD.
Bright Light Therapy in Parkinson’s Disease: An Overview of the Background and
Evidence. Parkinson’s Disease. 2012 Dec 23;2012:e767105.
38. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact
on quality of life in Parkinson’s disease - Barone - 2009 - Movement Disorders.
39. Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, et al.
Regulation of Monoamine Oxidase A by Circadian-Clock Components Implies Clock
Influence on Mood. Current Biology. 2008 May 6;18(9):678–83.
40. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in
the rat dorsal striatum via daily activation of D2 dopamine receptors.
41. Sakamoto K, Liu C, Kasamatsu M, Pozdeyev NV, Iuvone PM, Tosini G. Dopamine
regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion
cells. Eur J Neurosci. 2005 Dec;22(12):3129–36.
42. Lauretti E, Di Meco A, Merali S, Praticò D. Circadian rhythm dysfunction: a novel
environmental risk factor for Parkinson’s disease. Mol Psychiatry. 2017 Feb;22(2):280–6.
43. Videnovic A, Klerman EB, Wang W, Marconi A, Kuhta T, Zee PC. Timed Light Therapy
for Sleep and Daytime Sleepiness Associated With Parkinson Disease: A Randomized
Clinical Trial. JAMA Neurology. 2017 Apr 1;74(4):411–8.
44. Fifel K, Videnovic A. Chronotherapies for Parkinson’s disease. Progress in Neurobiology.
2019 Mar 1;174:16–27.
45. College of Optometry [Internet]. 2016 [cited 2023 Dec 28]. Scientists study effects of
sunlight to reduce number of nearsighted kids.
46. Isaac W. A study of the relationship between the visual system and the effects of
d-amphetamine. Physiology & Behavior. 1971 Feb 1;6(2):157–9.
47. Kallman WM, Isaac W. The effects of age and illumination on the dose-response curves
for three stimulants. Psychopharmacologia. 1975 Dec 1;40(4):313–8.
48. Pum ME, Rubio AR, Carey RJ, Silva MADS, Müller CP. The effects of cocaine on
light-induced activity. Brain Res Bull. 2011 Feb 28;84(3):229–34.
49. Willis GL. Intraocular microinjections repair experimental Parkinson’s disease. Brain
Research. 2008 Jun 27;1217:119–31.
50. Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, et al. Circadian Melatonin
Rhythm and Excessive Daytime Sleepiness in Parkinson Disease. JAMA Neurology.
2014 Apr 1;71(4):463–9.
51. Naskar A, Prabhakar V, Singh R, Dutta D, Mohanakumar KP. Melatonin enhances
L-DOPA therapeutic effects, helps to reduce its dose, and protects dopaminergic
neurons in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice.
Journal of Pineal Research. 2015;58(3):262–74.
52. Sun J, Lin XM, Lu DH, Wang M, Li K, Li SR, et al. Midbrain dopamine oxidation links
ubiquitination of glutathione peroxidase 4 to ferroptosis of dopaminergic neurons.
53. Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain
dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and
neurochemical correlations. J Neurol Sci. 1973 Dec;20(4):415–55.
54. Willis GL. Parkinson’s disease as a neuroendocrine disorder of circadian function:
dopamine-melatonin imbalance and the visual system in the genesis and progression of
the degenerative process. Rev Neurosci. 2008;19(4–5):245–316.
• • •
Missing some Tweet in this thread? You can try to
force a refresh
















