Since more people than ever struggle with candida overgrowths, here's the most comprehensive thread you've ever read on it.
We will discuss topics such as:
-What candida even is
-Common signs of candida overgrowth and why they actually appear
-Tests you can take
-The most common causes in order to finally determine the main ones that are driving your overgrowth
-How to address each one in the most efficient way possible
-Common mistakes to avoid
-Breakdown of the most helpful supplements in this journey
and more.
Thread🧵

We will discuss topics such as:
-What candida even is
-Common signs of candida overgrowth and why they actually appear
-Tests you can take
-The most common causes in order to finally determine the main ones that are driving your overgrowth
-How to address each one in the most efficient way possible
-Common mistakes to avoid
-Breakdown of the most helpful supplements in this journey
and more.
Thread🧵
*Standard disclaimer that nothing in this thread should be used as a substitute for medical advice*
It's George.
Candida is a genus of yeast that includes close to 200 species (not all of which are pathogenic).
These organisms are naturally found in and on the human body, particularly in the skin, nasal passages, digestive tract (mouth, esophagus, stomach, small intestine, large intestine etc) and female reproductive system.
In small amounts, Candida species are typically harmless and may even play beneficial roles in the gut microbiome.
However, an overgrowth of certain pathogenic species, particularly Candida albicans, can lead to infections known as candidiasis, ranging from superficial issues like oral thrush to life-threatening systemic infections.
It's George.
Candida is a genus of yeast that includes close to 200 species (not all of which are pathogenic).
These organisms are naturally found in and on the human body, particularly in the skin, nasal passages, digestive tract (mouth, esophagus, stomach, small intestine, large intestine etc) and female reproductive system.
In small amounts, Candida species are typically harmless and may even play beneficial roles in the gut microbiome.
However, an overgrowth of certain pathogenic species, particularly Candida albicans, can lead to infections known as candidiasis, ranging from superficial issues like oral thrush to life-threatening systemic infections.
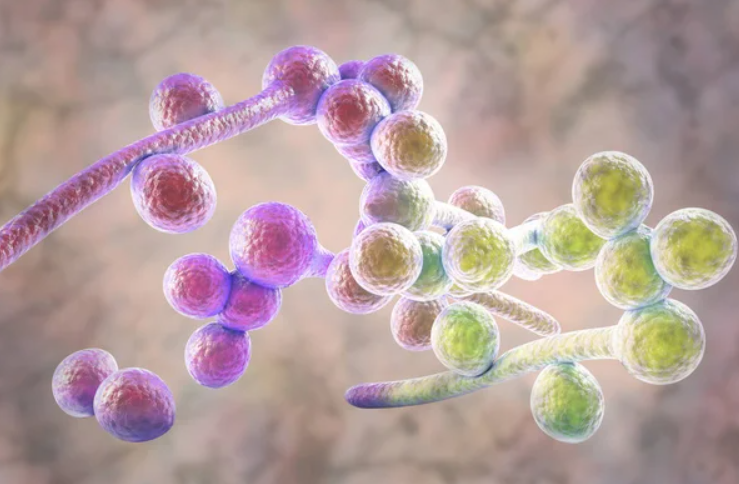
Overall, some potential signs and symptoms of Candida overgrowth include:
-Digestive issues such as bloating, constipation, white coating on the tongue or gas (especially after eating carbs).
-Brain fog that, even though it might sound weird, it’s very similar to a low-grade hangover (since it releases acetaldehyde) which is experienced once again, especially after eating carbs.
-Blood sugar regulation issues.
-Intense sugar cravings.
-Athlete’s foot, toenail fungus, jock itch, oral thrush, bad breath, acne and eczema.
-Chronic fatigue.
-Frequent infections.
-Leaky gut.
-Developing more and more food intolerances.
-Digestive issues such as bloating, constipation, white coating on the tongue or gas (especially after eating carbs).
-Brain fog that, even though it might sound weird, it’s very similar to a low-grade hangover (since it releases acetaldehyde) which is experienced once again, especially after eating carbs.
-Blood sugar regulation issues.
-Intense sugar cravings.
-Athlete’s foot, toenail fungus, jock itch, oral thrush, bad breath, acne and eczema.
-Chronic fatigue.
-Frequent infections.
-Leaky gut.
-Developing more and more food intolerances.
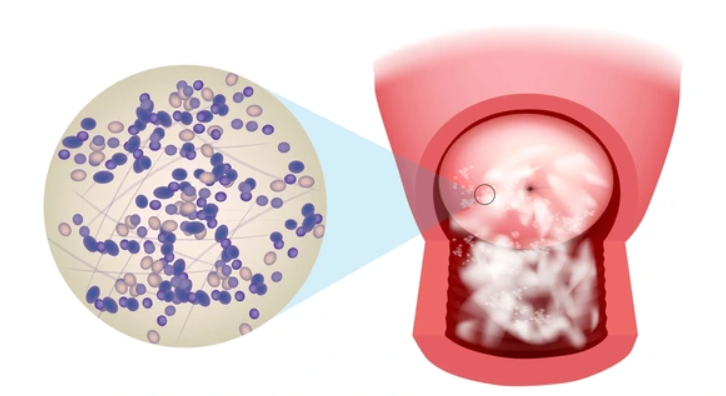
Now, how can a candida overgrowth lead to these symptoms?
Here are some basic explanations.
First, it disrupts the balance of gut microbiota, reducing beneficial bacteria.
This leads to fermentation of undigested carbohydrates, producing gas and bloating by 2–3-fold as most studies suggest.
It also secretes aspartyl proteases and phospholipases, damaging the mucosal bilayer which can lead to bloating and general discomfort.
When it comes to the oral cavity, it forms biofilms creating a white coating on the tongue.
Then, it metabolizes sugars via fermentation, producing acetaldehyde, a toxic byproduct that crosses the blood-brain barrier and impairs neuronal function, causing brain fog, confusion, and a “hangover-like” feeling.
In animal models for example, candida-induced inflammation reduced cognitive clarity by 30%.
Candida also consumes glucose for growth and biofilm formation, causing fluctuations in blood sugar levels, especially after high-carb meals.
In diabetic patients for example, an ovegrowth led to a 2-fold increase in insulin resistance.
Regarding sugar cravings, candida albicans thrives on glucose and its overgrowth may signal the host to consume more sugars throygh gut-brain axis modulation of hormones that control our appetite such as ghrelin.
Many studies show that a Candida overgrowth increased sugar cravingsand ghrelin levels in 20–30% of patients.
Fatigue-wise, candida overgrowth triggers cytokines such as IL-6 and TNF-α, causing systemic inflammation that disrupts energy metabolism, the acetaldehyde impairs mitochondrial function, reducing ATP production, biofilms and mucosal damage impair nutrient absorption (especially when it comes to B vitamins and iron), which are critical for energy production.
Then, candida overgrowth overwhelms mucosal immunity, reducing IgA and phagocytic activity, increasing susceptibility to bacterial and fungal infections.
And finally when it comes to effects such as developing a leaky gut and food intolerances, the overgrowth degrades tight junction proteins such as occludin and ZO-1 (it’s shown to reduce ZO-1 expression by 40%), increasing intestinal permeability allowing toxins and antigens to leak into the bloodstream triggering immune responses and food intolerances (Candida-induced leaky gut has shown to lead to a 30% increase in food intolerances).
Then of course, we have more rare symptoms that are extremely multifactorial such as anhedonia, histamine and oxalate intolerance.
The latter should not be used as tools for self-diagnosis since once again they are multifactorial and even though candida may be a contributor, it’s usually not a primary cause unless we are talking about chronic unaddressed infections.
Can a chronic candida albicans overgrowth for example deplete B vitamins such as B1 and B6 while increasing oxalic acid so much to the point of an oxalate \and even sulfur) intolerance?
Yes.
Can it also release so much acetaldehyde and thus salsolinol all the way to making someone experience symptoms of anhedonia?
Also yes.
But is it misleading to include these as the primary symptoms of a candida overgrowth out of context?
Also yes, at least in my opinion.
(*) Always remember that all symptoms are multifactorial.
So if you have a lot of them, it’s in fact true that the probability of you having a particular issue increases, but it does not mean that you have it even if you think “that’s me” every time you read a symptom.
Point being: Always test (there are test presented later) because self diagnosis a lot of the time is false and will lead you to follow the wrong roadmap which will only make the real unaddressed problem worse in the long run.
Now besides Candida albicans, other notable species of the Candida genus include Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei, which collectively account for 90-95% of infections.
Emerging pathogens like Candida auris are also concerning due to their multidrug resistance.
Here are some basic explanations.
First, it disrupts the balance of gut microbiota, reducing beneficial bacteria.
This leads to fermentation of undigested carbohydrates, producing gas and bloating by 2–3-fold as most studies suggest.
It also secretes aspartyl proteases and phospholipases, damaging the mucosal bilayer which can lead to bloating and general discomfort.
When it comes to the oral cavity, it forms biofilms creating a white coating on the tongue.
Then, it metabolizes sugars via fermentation, producing acetaldehyde, a toxic byproduct that crosses the blood-brain barrier and impairs neuronal function, causing brain fog, confusion, and a “hangover-like” feeling.
In animal models for example, candida-induced inflammation reduced cognitive clarity by 30%.
Candida also consumes glucose for growth and biofilm formation, causing fluctuations in blood sugar levels, especially after high-carb meals.
In diabetic patients for example, an ovegrowth led to a 2-fold increase in insulin resistance.
Regarding sugar cravings, candida albicans thrives on glucose and its overgrowth may signal the host to consume more sugars throygh gut-brain axis modulation of hormones that control our appetite such as ghrelin.
Many studies show that a Candida overgrowth increased sugar cravingsand ghrelin levels in 20–30% of patients.
Fatigue-wise, candida overgrowth triggers cytokines such as IL-6 and TNF-α, causing systemic inflammation that disrupts energy metabolism, the acetaldehyde impairs mitochondrial function, reducing ATP production, biofilms and mucosal damage impair nutrient absorption (especially when it comes to B vitamins and iron), which are critical for energy production.
Then, candida overgrowth overwhelms mucosal immunity, reducing IgA and phagocytic activity, increasing susceptibility to bacterial and fungal infections.
And finally when it comes to effects such as developing a leaky gut and food intolerances, the overgrowth degrades tight junction proteins such as occludin and ZO-1 (it’s shown to reduce ZO-1 expression by 40%), increasing intestinal permeability allowing toxins and antigens to leak into the bloodstream triggering immune responses and food intolerances (Candida-induced leaky gut has shown to lead to a 30% increase in food intolerances).
Then of course, we have more rare symptoms that are extremely multifactorial such as anhedonia, histamine and oxalate intolerance.
The latter should not be used as tools for self-diagnosis since once again they are multifactorial and even though candida may be a contributor, it’s usually not a primary cause unless we are talking about chronic unaddressed infections.
Can a chronic candida albicans overgrowth for example deplete B vitamins such as B1 and B6 while increasing oxalic acid so much to the point of an oxalate \and even sulfur) intolerance?
Yes.
Can it also release so much acetaldehyde and thus salsolinol all the way to making someone experience symptoms of anhedonia?
Also yes.
But is it misleading to include these as the primary symptoms of a candida overgrowth out of context?
Also yes, at least in my opinion.
(*) Always remember that all symptoms are multifactorial.
So if you have a lot of them, it’s in fact true that the probability of you having a particular issue increases, but it does not mean that you have it even if you think “that’s me” every time you read a symptom.
Point being: Always test (there are test presented later) because self diagnosis a lot of the time is false and will lead you to follow the wrong roadmap which will only make the real unaddressed problem worse in the long run.
Now besides Candida albicans, other notable species of the Candida genus include Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei, which collectively account for 90-95% of infections.
Emerging pathogens like Candida auris are also concerning due to their multidrug resistance.
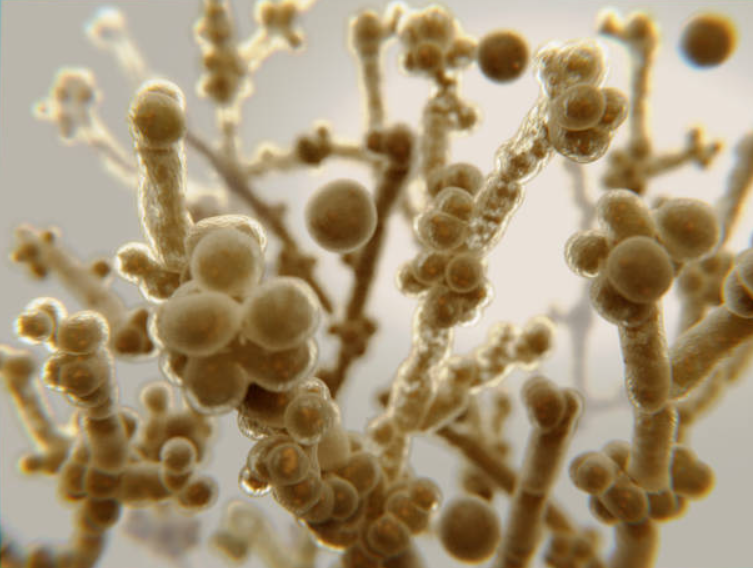
What’s also worth knowing from the get-go, is that candida albicans is a dimorphic fungus, existing in two primary forms:
-Yeast form: Single, oval-shaped cells ideal for dissemination through the bloodstream.
-Hyphal form: Elongated, thread-like filaments that facilitate tissue invasion and adherence to host cells.
This yeast-to-hypha transition is a major virulence factor, regulated by environmental cues like temperature (37°C favors hyphae), pH, CO2 levels, and nutrient availability. Key transcription factors, such as Efg1 and Ace2, and signaling pathways like cAMP-PKA and MAPK, orchestrate this process.
When it comes to the cell structure of candida albicans, it has a quite complex anatomy with each component helping it to adapt, colozine, invade and resist our immune defenses but also antifungal treatments to a certain extent.
-Yeast form: Single, oval-shaped cells ideal for dissemination through the bloodstream.
-Hyphal form: Elongated, thread-like filaments that facilitate tissue invasion and adherence to host cells.
This yeast-to-hypha transition is a major virulence factor, regulated by environmental cues like temperature (37°C favors hyphae), pH, CO2 levels, and nutrient availability. Key transcription factors, such as Efg1 and Ace2, and signaling pathways like cAMP-PKA and MAPK, orchestrate this process.
When it comes to the cell structure of candida albicans, it has a quite complex anatomy with each component helping it to adapt, colozine, invade and resist our immune defenses but also antifungal treatments to a certain extent.
Also, even though its anatomy differs slightly between yeast and hyphal forms, certain key components are consistent such as:
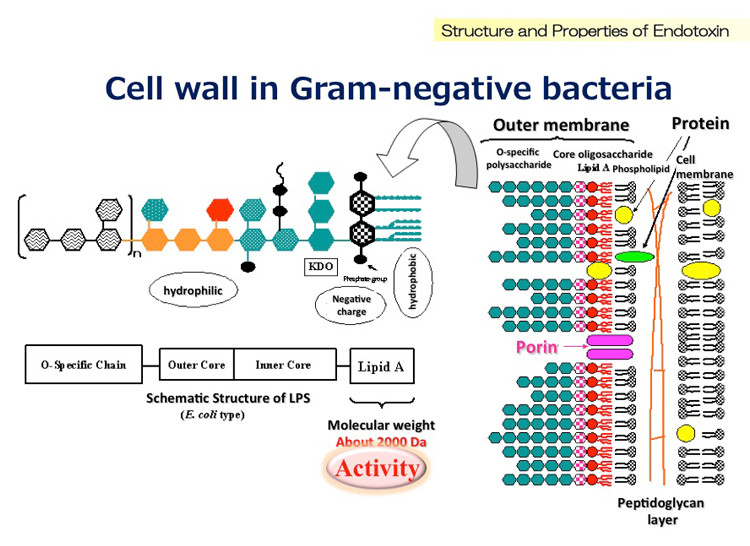
1. The cell wall
This is a multi-layered structure that provides structural integrity and serves as the first line of defense against environmental stresses (think antifungals) and immune attacks.
It's composed out of:
-β-Glucans (30–50%)
These are branched polysaccharides (primarily β-1,3- and β-1,6-glucans) that form the structural scaffold of the cell wall supporting cell integrity under stress.
-Chitin (1–7%)
This polysaccharide reinforces the cell wall’s structural integrity.
-Mannoproteins (30–40%)
These glycoproteins, located on the outer layer, are pivotal for host-pathogen interactions.
-Proteins like ALS1, ALS3, and HWP1 mediate adhesion to host tissues, such as mucosal surfaces.
Mannoproteins also reduce immune recognition by up to 50% by shielding β-glucans from dectin-1 receptors on macrophages and neutrophils, thus reducing phagocytosis.
-Lipids
Though a minor component and lipids anchor the cell wall to the underlying plasma membrane, contributing to structural stability as well.
2. The cell membrane
Beneath the cell wall lies the cell membrane which is a lipid bilayer composed primarily of phospholipids and ergosterol that regulates the transport of ions, nutrients, and waste, maintaining cellular balance.
Ergosterol is a fungal-specific sterol that distinguishes fungal membranes from cholesterol-containing human membranes (it basically stabilizes the membrane’s fluidity and structure).
For example, fluconazole’s disruption of ergosterol synthesis increased membrane permeability by 70%.
Also, embedded within are proteins like efflux pumps (CDR1, CDR2, MDR1 for example) and enzymes such as lanosterol 14-α-demethylase (encoded by ERG11), which are critical for membrane function and antifungal resistance.
These proteins actively expel antifungal compounds before they can disrupt membranes or induce ROS, thus reducing their intracellular concentration.
To put in perspective how important these are for the survival of the cells, the overexpression of CDR1 and CDR2 efflux pumps in resistant strains reduced azole efficacy by up to 40%.
Not only that, but CDR1 overexpression has been shown to reduce berberine efficacy by 50% and oregano oil’s potency by 40%.
3. The cytoplasm
The cytoplasm of C. albicans contains organelles that drive essential cellular and metabolic processes like glycolysis, protein synthesis and lipid production, which are essential for growth and virulence.
These organelles include:
-Nucleus
This one houses a diploid genome encoding genes for virulence factors such as adhesins, secreted aspartyl proteases and antifungal resistance (ERG11, CDR1).
It tightly regulates gene expression in response to environmental cues, such as pH or nutrient availability.
-Mitochondria
The well known powerhouse of the cell.
They are targeted by antifungals like berberine (berberine’s induction of mitochondrial ROS reduced ATP levels by 70%, thus being very effective at harming C. albicans metabolism) which induce excessive ROS to disrupt energy production and trigger cell death.
-Endoplasmic eeticulum (ER)
The ER synthesizes lipids such as ergosterol and proteins, folding them into functional forms. and it also detoxifies harmful compounds.
-Vacuoles
These organelles store nutrients, degrade waste, and maintain pH homeostasis.
So they are crucial for survival during nutrient scarcity or stress, such as exposure to antifungals.
4. Hyphal structures
Candida albicans switches between yeast and hyphal forms in response to environmental cues.
Hyphal forms have thicker cell walls and altered membrane composition, reducing susceptibility to antifungals (they have a 3-fold lower susceptibility to membrane targeting agents).
Genes like EFG1, Cph1, and HWP1 control the yeast-to-hyphal transition.
Unlike yeast forms, hyphae lack bud scars and are specialized for tissue invasion and pathogenesis while having a higher chitin and glucan content.
Septa divide the hyphae into compartments, maintaining structural integrity during elongation.
5. The biofilm matrix
Probably the most famous protective mechanism and for good reasons since for example they have been shown to reduce antifungal penetration by 60% (but don’t worry since berberine has been shown to reduce biofilm biomass by up to 70% through downregulating ALS3).
Biofilms create a physical barrier that limits penetration of antifungals and immune cells.
The matrix sequesters drugs, while persister cells within biofilms survive high doses.
Biofilms also upregulate efflux pumps and stress response genes.
When C. albicans forms biofilms, it creates a complex extracellular matrix composed of β-glucans, proteins, and extracellular DNA (eDNA).
This matrix encases yeast and hyphal cells, forming a protective barrier on surfaces like mucosal tissues or medical devices.
Also quorum-sensing molecules (more about this later) like farnesol regulate biofilm development and dispersal, coordinating cellular behavior.

1. The cell wall
This is a multi-layered structure that provides structural integrity and serves as the first line of defense against environmental stresses (think antifungals) and immune attacks.
It's composed out of:
-β-Glucans (30–50%)
These are branched polysaccharides (primarily β-1,3- and β-1,6-glucans) that form the structural scaffold of the cell wall supporting cell integrity under stress.
-Chitin (1–7%)
This polysaccharide reinforces the cell wall’s structural integrity.
-Mannoproteins (30–40%)
These glycoproteins, located on the outer layer, are pivotal for host-pathogen interactions.
-Proteins like ALS1, ALS3, and HWP1 mediate adhesion to host tissues, such as mucosal surfaces.
Mannoproteins also reduce immune recognition by up to 50% by shielding β-glucans from dectin-1 receptors on macrophages and neutrophils, thus reducing phagocytosis.
-Lipids
Though a minor component and lipids anchor the cell wall to the underlying plasma membrane, contributing to structural stability as well.
2. The cell membrane
Beneath the cell wall lies the cell membrane which is a lipid bilayer composed primarily of phospholipids and ergosterol that regulates the transport of ions, nutrients, and waste, maintaining cellular balance.
Ergosterol is a fungal-specific sterol that distinguishes fungal membranes from cholesterol-containing human membranes (it basically stabilizes the membrane’s fluidity and structure).
For example, fluconazole’s disruption of ergosterol synthesis increased membrane permeability by 70%.
Also, embedded within are proteins like efflux pumps (CDR1, CDR2, MDR1 for example) and enzymes such as lanosterol 14-α-demethylase (encoded by ERG11), which are critical for membrane function and antifungal resistance.
These proteins actively expel antifungal compounds before they can disrupt membranes or induce ROS, thus reducing their intracellular concentration.
To put in perspective how important these are for the survival of the cells, the overexpression of CDR1 and CDR2 efflux pumps in resistant strains reduced azole efficacy by up to 40%.
Not only that, but CDR1 overexpression has been shown to reduce berberine efficacy by 50% and oregano oil’s potency by 40%.
3. The cytoplasm
The cytoplasm of C. albicans contains organelles that drive essential cellular and metabolic processes like glycolysis, protein synthesis and lipid production, which are essential for growth and virulence.
These organelles include:
-Nucleus
This one houses a diploid genome encoding genes for virulence factors such as adhesins, secreted aspartyl proteases and antifungal resistance (ERG11, CDR1).
It tightly regulates gene expression in response to environmental cues, such as pH or nutrient availability.
-Mitochondria
The well known powerhouse of the cell.
They are targeted by antifungals like berberine (berberine’s induction of mitochondrial ROS reduced ATP levels by 70%, thus being very effective at harming C. albicans metabolism) which induce excessive ROS to disrupt energy production and trigger cell death.
-Endoplasmic eeticulum (ER)
The ER synthesizes lipids such as ergosterol and proteins, folding them into functional forms. and it also detoxifies harmful compounds.
-Vacuoles
These organelles store nutrients, degrade waste, and maintain pH homeostasis.
So they are crucial for survival during nutrient scarcity or stress, such as exposure to antifungals.
4. Hyphal structures
Candida albicans switches between yeast and hyphal forms in response to environmental cues.
Hyphal forms have thicker cell walls and altered membrane composition, reducing susceptibility to antifungals (they have a 3-fold lower susceptibility to membrane targeting agents).
Genes like EFG1, Cph1, and HWP1 control the yeast-to-hyphal transition.
Unlike yeast forms, hyphae lack bud scars and are specialized for tissue invasion and pathogenesis while having a higher chitin and glucan content.
Septa divide the hyphae into compartments, maintaining structural integrity during elongation.
5. The biofilm matrix
Probably the most famous protective mechanism and for good reasons since for example they have been shown to reduce antifungal penetration by 60% (but don’t worry since berberine has been shown to reduce biofilm biomass by up to 70% through downregulating ALS3).
Biofilms create a physical barrier that limits penetration of antifungals and immune cells.
The matrix sequesters drugs, while persister cells within biofilms survive high doses.
Biofilms also upregulate efflux pumps and stress response genes.
When C. albicans forms biofilms, it creates a complex extracellular matrix composed of β-glucans, proteins, and extracellular DNA (eDNA).
This matrix encases yeast and hyphal cells, forming a protective barrier on surfaces like mucosal tissues or medical devices.
Also quorum-sensing molecules (more about this later) like farnesol regulate biofilm development and dispersal, coordinating cellular behavior.

More on the protective mechanisms (very important to understand).
Now Candida albicans employs several protective mechanisms to evade host immune responses and antifungal treatments.
We saw 3 of them so far: Efflux pumps, biofilm formation and morphological switching.
But there are more, such as:
-Antioxidant defenses
Candida for example produces antioxidant enzymes such as superoxide dismutase [SOD1] and catalase [CAT1] to neutralize the efficacy of ROS-inducing agents like berberine, oregano oil, or allicin.
SOD1 overexpression for example has been shown to decrease berberine’s ROS-mediated killing by 50% (but once again don’t worry since we have tools for working around this as well).
The Hsp90 pathway also repairs oxidative damage.
-Enzymatic degradation
Candida produces enzymes like lipases, esterases, and cytochrome P450 that degrade or modify antifungal compounds.
Lipases for example break down fatty acids like caprylic acid or undecylenic acid, reducing their potency by up to 40% ot cytochrome P450 enzymes metabolize berberine and lower its concentration.
-Target site alterations
Mutations in target genes such as ERG11 for ergosterol synthesis alter binding sites, reducing antifungal efficacy.
For example, ERG11 mutations decrease berberine and oregano oil’s ability to disrupt membranes by up to 30%.
But once again, we have ways of working around this. Allicin for example targets thiol groups rather than ergosterol.
-Immune evasion
We briefly talked about this where mannoproteins in the cell wall mask β-glucans, reducing recognition by immune receptors like dectin-1.
But candida also secretes aspartyl proteases that degrade host immune proteins.
That’s partly why if we want to effectively manage candida we need to enhance immune responses via IgA production or macrophage activation.
Now Candida albicans employs several protective mechanisms to evade host immune responses and antifungal treatments.
We saw 3 of them so far: Efflux pumps, biofilm formation and morphological switching.
But there are more, such as:
-Antioxidant defenses
Candida for example produces antioxidant enzymes such as superoxide dismutase [SOD1] and catalase [CAT1] to neutralize the efficacy of ROS-inducing agents like berberine, oregano oil, or allicin.
SOD1 overexpression for example has been shown to decrease berberine’s ROS-mediated killing by 50% (but once again don’t worry since we have tools for working around this as well).
The Hsp90 pathway also repairs oxidative damage.
-Enzymatic degradation
Candida produces enzymes like lipases, esterases, and cytochrome P450 that degrade or modify antifungal compounds.
Lipases for example break down fatty acids like caprylic acid or undecylenic acid, reducing their potency by up to 40% ot cytochrome P450 enzymes metabolize berberine and lower its concentration.
-Target site alterations
Mutations in target genes such as ERG11 for ergosterol synthesis alter binding sites, reducing antifungal efficacy.
For example, ERG11 mutations decrease berberine and oregano oil’s ability to disrupt membranes by up to 30%.
But once again, we have ways of working around this. Allicin for example targets thiol groups rather than ergosterol.
-Immune evasion
We briefly talked about this where mannoproteins in the cell wall mask β-glucans, reducing recognition by immune receptors like dectin-1.
But candida also secretes aspartyl proteases that degrade host immune proteins.
That’s partly why if we want to effectively manage candida we need to enhance immune responses via IgA production or macrophage activation.
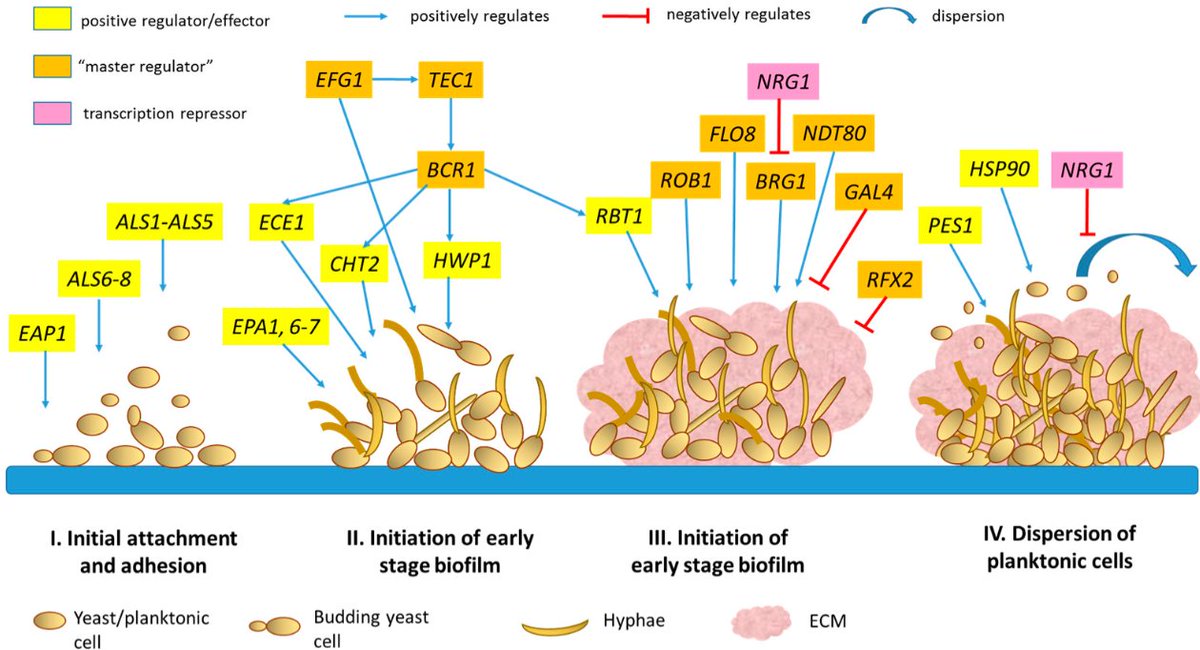
Now the KEY genes in candida albicans include the following.
Each of these genes plays a distinct role in Candida albicans’ ability to colonize, invade, persist, and resist treatment.
Down below you will find the main role of each one summed up and the most effective OTC tools.
1. ERG11 (Lanosterol 14-α-Demethylase)
This encodes lanosterol 14-α-demethylase (a cytochrome P450 enzyme) essential for ergosterol synthesis (a component of the fungal cell membrane).
*Berberine binds to the heme-binding site or substrate pocket of lanosterol 14-α-demethylase, inhibiting its catalytic activity. This disrupts ergosterol synthesis and increases membrane permeability
**Alycin targets thiol groups, bypassing ERG11-mediated resistance/ERG11-resistant strains.
2. CDR1 (Candida Drug Resistance 1)
This encodes an ATP-binding cassette (ABC) efflux pump that expels a wide range of antifungals reducing their intracellular concentration.
*Berberine binds to CDR1’s substrate-binding site, competitively inhibiting its efflux activity, increasing intracellular antifungal concentrations.
**Quercetin inhibits CDR1 by binding to their nucleotide-binding domains, reducing ATP hydrolysis and efflux activity (this increases intracellular concentrations of antifungals).
***CDR1 expression is 3–5-fold higher in biofilms due to hypoxia, nutrient limitation, and quorum-sensing signals. Serrapeptase degrades biofilm extracellular matrix (ECM) components like proteins and extracellular DNA (eDNA), disrupting the hypoxic microenvironment that upregulates CDR1.
3. ALS3 (Agglutinin-Like Sequence 3)
This encodes a cell wall adhesin, a glycoprotein critical for initiating biofilm formation and tissue invasion.
*Bifidobacterium bifidum competes with Candida albicans for binding sites on mucosal surfaces, reducing ALS3-mediated adhesion.
**Berberine inhibits ALS3 expression by targeting Efg1 and Bcr1 transcription factors.
***Low-ish carb diet which limits glucose availability thus decreasing ALS3 expression by limiting Efg1 and Bcr1 activity (overall, a low-sugar diet reduces Candida biofilm formation by 2–3-fold in most studies).
****Oregano oil downregulates ALS3 (can downregulate its expression by up to 60%) and HWP1 by inhibiting Efg1, reducing adhesion and hyphal growth.
*****Allicin inhibits ALS3 by targeting thiol groups in adhesin proteins, reducing adhesion and biofilm formation (it can reduce ALS3 expression by 40% and oral biofilms by 40%).
4. HWP1 (Hyphal Wall Protein 1)
This encodes a hyphal-specific cell wall protein that promotes adhesion to host tissues and facilitates biofilm formation.
*Caprylic acid inhibits lipases and enzymes required for hyphal morphogenesis, downregulating HWP1 expression (it can do so by up to 50%) by targeting the Efg1 pathway.
**Carvacrol can reduce HWP1 expression by 60%.
***Serrapeptase reduces HWP1-driven cell-cell adhesion decreasing HWP1 expression by 40% in a lot of studies.
5. BCR1 (Biofilm and Cell Wall Regulator 1)
This one encodes a transcription factor that regulates biofilm formation by controlling expression of adhesins (ALS3, HWP1) and extracellular matrix genes.
It basically romotes biofilm formation, adhesion and hyphal growth.
*Berberine inhibits BCR1 expression (by up to 50%) by targeting Efg1 and other transcription factors, reducing ALS3 and HWP1 expression, which weakens biofilm formation.
**Serrapeptase degrades ECM proteins and eDNA, reducing BCR1-driven adhesion by 40%.
***Lactobacillus rhamnosus reduces BCR1 expression by 50%.
6. EFG1 (Enhanced Filamentous Growth 1)
This one encodes a transcription factor in the cAMP-PKA pathway thus regulating hyphal growth, biofilm formation and the expression of ALS3 and HWP1.
*Caprylic acid inhibits lipases and enzymes required for hyphal morphogenesis, downregulating EFG1 expression by targeting the cAMP-PKA pathway.
**Undecylenic acid reduces EFG1 expression by 40%.
***Berberine inhibits the cAMP-PKA pathway, reducing EFG1 expression.
7. SAP Genes (Secreted Aspartyl Proteinases (SAP1–SAP10))
Encode a family of secreted aspartyl proteases that degrade host proteins such as mucins, collagen and immune factors like IgA.
*Probiotics such as Bifidobacterium bifidum increase mucosal IgA production, countering SAP-mediated degradation of IgA and enhancing immune clearance.
**Tributyrin increases MUC2 expression and strengthening the mucosal bilayer, reducing SAP-driven damage.
***Allicin inhibits SAP activity by reacting with thiol groups in aspartyl proteases, reducing their ability to degrade mucins and IgA.
8. SOD1 (Superoxide Dismutase 1)
This encodes a superoxide dismutase enzyme that neutralizes reactive oxygen species (ROS) generated by antifungals like berberine, oregano oil, and garlic, protecting Candida from oxidative stress.
*High dose berberine (just because it's more well tolerated than high dose oregano oil) that generates high levels of ROS, exceeding SOD1’s capacity to neutralize superoxide radicals.
**Regular dose of berberine combined with NAC since NAC enhances oregano oil’s efficacy by 50% by overwhelming SOD1 when used together.
***Curcumin can induce ROS in fungal cells by disrupting mitochondrial function and increasing oxidative stress.
****Blackseed oil/Thymoquinone also overwhelms SOD1 defenses, reducing Candida’s ability to neutralize ROS, leading to apoptosis-like cell death.
Note: In case it’s unclear, certain compounds can boost reactive oxygen species (ROS) production in fungal cells while simultaneously supporting host immunity.
Each of these genes plays a distinct role in Candida albicans’ ability to colonize, invade, persist, and resist treatment.
Down below you will find the main role of each one summed up and the most effective OTC tools.
1. ERG11 (Lanosterol 14-α-Demethylase)
This encodes lanosterol 14-α-demethylase (a cytochrome P450 enzyme) essential for ergosterol synthesis (a component of the fungal cell membrane).
*Berberine binds to the heme-binding site or substrate pocket of lanosterol 14-α-demethylase, inhibiting its catalytic activity. This disrupts ergosterol synthesis and increases membrane permeability
**Alycin targets thiol groups, bypassing ERG11-mediated resistance/ERG11-resistant strains.
2. CDR1 (Candida Drug Resistance 1)
This encodes an ATP-binding cassette (ABC) efflux pump that expels a wide range of antifungals reducing their intracellular concentration.
*Berberine binds to CDR1’s substrate-binding site, competitively inhibiting its efflux activity, increasing intracellular antifungal concentrations.
**Quercetin inhibits CDR1 by binding to their nucleotide-binding domains, reducing ATP hydrolysis and efflux activity (this increases intracellular concentrations of antifungals).
***CDR1 expression is 3–5-fold higher in biofilms due to hypoxia, nutrient limitation, and quorum-sensing signals. Serrapeptase degrades biofilm extracellular matrix (ECM) components like proteins and extracellular DNA (eDNA), disrupting the hypoxic microenvironment that upregulates CDR1.
3. ALS3 (Agglutinin-Like Sequence 3)
This encodes a cell wall adhesin, a glycoprotein critical for initiating biofilm formation and tissue invasion.
*Bifidobacterium bifidum competes with Candida albicans for binding sites on mucosal surfaces, reducing ALS3-mediated adhesion.
**Berberine inhibits ALS3 expression by targeting Efg1 and Bcr1 transcription factors.
***Low-ish carb diet which limits glucose availability thus decreasing ALS3 expression by limiting Efg1 and Bcr1 activity (overall, a low-sugar diet reduces Candida biofilm formation by 2–3-fold in most studies).
****Oregano oil downregulates ALS3 (can downregulate its expression by up to 60%) and HWP1 by inhibiting Efg1, reducing adhesion and hyphal growth.
*****Allicin inhibits ALS3 by targeting thiol groups in adhesin proteins, reducing adhesion and biofilm formation (it can reduce ALS3 expression by 40% and oral biofilms by 40%).
4. HWP1 (Hyphal Wall Protein 1)
This encodes a hyphal-specific cell wall protein that promotes adhesion to host tissues and facilitates biofilm formation.
*Caprylic acid inhibits lipases and enzymes required for hyphal morphogenesis, downregulating HWP1 expression (it can do so by up to 50%) by targeting the Efg1 pathway.
**Carvacrol can reduce HWP1 expression by 60%.
***Serrapeptase reduces HWP1-driven cell-cell adhesion decreasing HWP1 expression by 40% in a lot of studies.
5. BCR1 (Biofilm and Cell Wall Regulator 1)
This one encodes a transcription factor that regulates biofilm formation by controlling expression of adhesins (ALS3, HWP1) and extracellular matrix genes.
It basically romotes biofilm formation, adhesion and hyphal growth.
*Berberine inhibits BCR1 expression (by up to 50%) by targeting Efg1 and other transcription factors, reducing ALS3 and HWP1 expression, which weakens biofilm formation.
**Serrapeptase degrades ECM proteins and eDNA, reducing BCR1-driven adhesion by 40%.
***Lactobacillus rhamnosus reduces BCR1 expression by 50%.
6. EFG1 (Enhanced Filamentous Growth 1)
This one encodes a transcription factor in the cAMP-PKA pathway thus regulating hyphal growth, biofilm formation and the expression of ALS3 and HWP1.
*Caprylic acid inhibits lipases and enzymes required for hyphal morphogenesis, downregulating EFG1 expression by targeting the cAMP-PKA pathway.
**Undecylenic acid reduces EFG1 expression by 40%.
***Berberine inhibits the cAMP-PKA pathway, reducing EFG1 expression.
7. SAP Genes (Secreted Aspartyl Proteinases (SAP1–SAP10))
Encode a family of secreted aspartyl proteases that degrade host proteins such as mucins, collagen and immune factors like IgA.
*Probiotics such as Bifidobacterium bifidum increase mucosal IgA production, countering SAP-mediated degradation of IgA and enhancing immune clearance.
**Tributyrin increases MUC2 expression and strengthening the mucosal bilayer, reducing SAP-driven damage.
***Allicin inhibits SAP activity by reacting with thiol groups in aspartyl proteases, reducing their ability to degrade mucins and IgA.
8. SOD1 (Superoxide Dismutase 1)
This encodes a superoxide dismutase enzyme that neutralizes reactive oxygen species (ROS) generated by antifungals like berberine, oregano oil, and garlic, protecting Candida from oxidative stress.
*High dose berberine (just because it's more well tolerated than high dose oregano oil) that generates high levels of ROS, exceeding SOD1’s capacity to neutralize superoxide radicals.
**Regular dose of berberine combined with NAC since NAC enhances oregano oil’s efficacy by 50% by overwhelming SOD1 when used together.
***Curcumin can induce ROS in fungal cells by disrupting mitochondrial function and increasing oxidative stress.
****Blackseed oil/Thymoquinone also overwhelms SOD1 defenses, reducing Candida’s ability to neutralize ROS, leading to apoptosis-like cell death.
Note: In case it’s unclear, certain compounds can boost reactive oxygen species (ROS) production in fungal cells while simultaneously supporting host immunity.
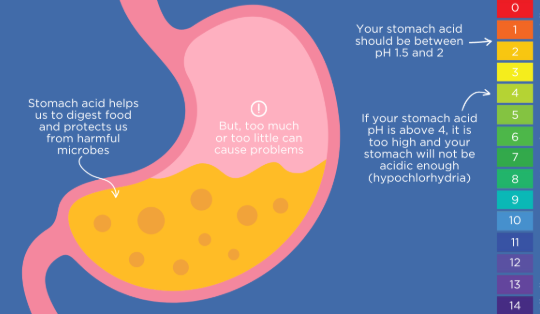
Now as stated, we are discussing an opportunistic microbe, so a good question to ask would be what environment allows these opportunistic microbes to grow.
The most common causes nowadays are:
-Low HCL
-Low transit time
-A compromised immune system
-Lack of antimicrobial peptides
-Poor bile flow
-Hormonal issues such as low thyroid hormones and/or insulin resistance
-Certain medications
-Unbound iron
-Malnutrition / the wrong dietary habits
-Environmental toxins such as heavy metals and xenoestrogens
-Unhealthy mast cells/histamine intolerance
The most common causes nowadays are:
-Low HCL
-Low transit time
-A compromised immune system
-Lack of antimicrobial peptides
-Poor bile flow
-Hormonal issues such as low thyroid hormones and/or insulin resistance
-Certain medications
-Unbound iron
-Malnutrition / the wrong dietary habits
-Environmental toxins such as heavy metals and xenoestrogens
-Unhealthy mast cells/histamine intolerance
Here's how:
Hydrochloric acid (HCl), produced by parietal cells in the stomach and performs certain critical functions such as:
-Killing ingested bacteria, fungi, and parasites by creating an acidic environment (pH 1.5–3.5 (the low pH denatures microbial proteins, disrupts cell membranes, and activates pepsin, a protease that further degrades pathogens)).
-Breaking down proteins into amino acids by denaturing them (unfolding their complex structures) and activating pepsin, which cleaves proteins into smaller peptides.
-Facilitating the absorption of nutrients such as vitamin B12, iron, and other minerals.
HCl for example releases B12 from dietary proteins, allowing it to bind to intrinsic factor (also secreted by parietal cells) and converts dietary ferric iron (Fe³⁺) to ferrous iron (Fe²⁺), which is more absorbable.
Triggering the release of secretin and cholecystokinin (CCK) from the duodenum, signaling the pancreas to secrete digestive enzymes (lipase, protease, amylase) and the gallbladder to release bile.
Now, regarding testing, the Heidelberg test is a precise but quite costly lab test.
Now here are some mechanisms by which slow transit time promotes a candida overgrowth.
First, slow transit time obviously allows undigested food to remain in the gut for a longer period.
This is especially problematic when it comes to simple sugars and carbohydrates.
Prolonged intestinal transit in mice for example has been shown to increase gut glucose levels, leading to a 3-fold rise in Candida albicans colonization (glucose activates the Ras1-cAMP-PKA pathway, increasing expression of adhesins such as Als1, Als3 and Hwp1 and quorum-sensing molecules, which enhance adhesion sites and biofilm formation).
Besides this, extended nutrient availability supports rapid budding and hyphal growth and reduces the turnover of bacteria such as Lactobacillus and Bifidobacterium, which normally compete with Candida for adhesion sites, nutrients and produce antifungal metabolites such as lactic acid or hydrogen peroxide that maintain an acidic gut pH (4.5–5.5), inhospitable to Candida.
A slow transit in IBS-C patients correlated with a 50% reduction in Lactobacillus diversity and a 2-fold increase in Candida colonization and there are other ones that show that slow transit in humans increased gut pH from 5.5 to 6.5, correlating with a 3-fold rise in Candida growth.
Slow transit can also compromise mucosal immunity by reducing the clearance of Candida cells and their antigens, allowing prolonged exposure to immune cells.
This may lead to immune tolerance or dysfunction, particularly in the gut-associated lymphoid tissue (GALT), where IgA and T-cells normally control Candida.
To put this in perspective, slow transit in mice can reduce mucosal IgA by 40%.
All these also, support the formation of biofilms and a slow intestinal transit in rats has been shown to increase Candida biofilm formation by 4-fold.
Now let’s move on to how a compromised immune system contributes to a Candida overgrowth.
First, neutrophils, macrophages and dendritic cells are critical for engulfing and destroying Candida cells through phagocytosis and obviously, a compromised immune system reduces phagocytic capacity.
For example, corticosteroid-induced neutropenia in mice increased systemic candidiasis risk by 4-fold due to impaired phagocytosis.
Then we have impaired antifungal cytokine production.
The immune system relies on cytokines like interleukin-17 (IL-17), IL-22, and interferon-gamma (IFN-γ) to coordinate antifungal responses.
These cytokines, produced by Th17 cells and innate lymphoid cells, enhance epithelial barrier function, stimulate antimicrobial peptide production and activate phagocytes.
Immunosuppression reduces cytokine production, weakening mucosal defenses.
IL-17 deficiencies for example in chronic mucocutaneous candidiasis increased Candida colonization by 3-fold.
Of course, we also have reduced mucosal IgA production, which is also key since secretory IgA (sIgA) in mucosal surfaces (think the gut, vagina, oral cavity) binds Candida cells, preventing adhesion and promoting clearance.
But a compromised immune system, reduces sIgA production, allowing Candida to colonize mucosal adhesion sites.
It will also lead to overall dysregulated inflammatory responses, either through excessive proinflammatory cytokines such as IL-1β or IL-6, or insufficient anti-inflammatory responses.
This is also a problem since for example excessive IL-6 suppresses Th17 responses and reduces IL-17.
Then let’s not forget that the immune system uses reactive oxygen species (ROS) produced by phagocytes (neutrophils via NADPH oxidase) to kill Candida.
So a compromised immune system reduces ROS production, allowing Candida to survive where reduced host ROS enables Candida to maintain thiol-dependent enzymes, supporting metabolism, adhesion, and biofilm formation.
This is why some studies show that that CGD patients with impaired ROS production have a 5-fold higher risk of systemic candidiasis.
Moving on to antimicrobial peptides (AMPs) since they are quite connected with our immune system.
These, directly kill microbes by disrupting cell membranes, inducing reactive oxygen species (ROS), or inhibiting metabolic pathways, modulate immune responses by recruiting immune cells such as neutrophils and macrophages and stimulating cytokine production (IL-17 and IL-22 for example).
To put in perspective how important these are, histatin-5 reduced Candida albicans viability by 80% in vitro, and its absence in salivary deficiencies increased oral thrush risk by 4-fold.
Histatin-5 can even inhibit Candida’s metabolic enzymes such as ADH by up to 60%, disrupting energy production and nutrient acquisition.
Hydrochloric acid (HCl), produced by parietal cells in the stomach and performs certain critical functions such as:
-Killing ingested bacteria, fungi, and parasites by creating an acidic environment (pH 1.5–3.5 (the low pH denatures microbial proteins, disrupts cell membranes, and activates pepsin, a protease that further degrades pathogens)).
-Breaking down proteins into amino acids by denaturing them (unfolding their complex structures) and activating pepsin, which cleaves proteins into smaller peptides.
-Facilitating the absorption of nutrients such as vitamin B12, iron, and other minerals.
HCl for example releases B12 from dietary proteins, allowing it to bind to intrinsic factor (also secreted by parietal cells) and converts dietary ferric iron (Fe³⁺) to ferrous iron (Fe²⁺), which is more absorbable.
Triggering the release of secretin and cholecystokinin (CCK) from the duodenum, signaling the pancreas to secrete digestive enzymes (lipase, protease, amylase) and the gallbladder to release bile.
Now, regarding testing, the Heidelberg test is a precise but quite costly lab test.
Now here are some mechanisms by which slow transit time promotes a candida overgrowth.
First, slow transit time obviously allows undigested food to remain in the gut for a longer period.
This is especially problematic when it comes to simple sugars and carbohydrates.
Prolonged intestinal transit in mice for example has been shown to increase gut glucose levels, leading to a 3-fold rise in Candida albicans colonization (glucose activates the Ras1-cAMP-PKA pathway, increasing expression of adhesins such as Als1, Als3 and Hwp1 and quorum-sensing molecules, which enhance adhesion sites and biofilm formation).
Besides this, extended nutrient availability supports rapid budding and hyphal growth and reduces the turnover of bacteria such as Lactobacillus and Bifidobacterium, which normally compete with Candida for adhesion sites, nutrients and produce antifungal metabolites such as lactic acid or hydrogen peroxide that maintain an acidic gut pH (4.5–5.5), inhospitable to Candida.
A slow transit in IBS-C patients correlated with a 50% reduction in Lactobacillus diversity and a 2-fold increase in Candida colonization and there are other ones that show that slow transit in humans increased gut pH from 5.5 to 6.5, correlating with a 3-fold rise in Candida growth.
Slow transit can also compromise mucosal immunity by reducing the clearance of Candida cells and their antigens, allowing prolonged exposure to immune cells.
This may lead to immune tolerance or dysfunction, particularly in the gut-associated lymphoid tissue (GALT), where IgA and T-cells normally control Candida.
To put this in perspective, slow transit in mice can reduce mucosal IgA by 40%.
All these also, support the formation of biofilms and a slow intestinal transit in rats has been shown to increase Candida biofilm formation by 4-fold.
Now let’s move on to how a compromised immune system contributes to a Candida overgrowth.
First, neutrophils, macrophages and dendritic cells are critical for engulfing and destroying Candida cells through phagocytosis and obviously, a compromised immune system reduces phagocytic capacity.
For example, corticosteroid-induced neutropenia in mice increased systemic candidiasis risk by 4-fold due to impaired phagocytosis.
Then we have impaired antifungal cytokine production.
The immune system relies on cytokines like interleukin-17 (IL-17), IL-22, and interferon-gamma (IFN-γ) to coordinate antifungal responses.
These cytokines, produced by Th17 cells and innate lymphoid cells, enhance epithelial barrier function, stimulate antimicrobial peptide production and activate phagocytes.
Immunosuppression reduces cytokine production, weakening mucosal defenses.
IL-17 deficiencies for example in chronic mucocutaneous candidiasis increased Candida colonization by 3-fold.
Of course, we also have reduced mucosal IgA production, which is also key since secretory IgA (sIgA) in mucosal surfaces (think the gut, vagina, oral cavity) binds Candida cells, preventing adhesion and promoting clearance.
But a compromised immune system, reduces sIgA production, allowing Candida to colonize mucosal adhesion sites.
It will also lead to overall dysregulated inflammatory responses, either through excessive proinflammatory cytokines such as IL-1β or IL-6, or insufficient anti-inflammatory responses.
This is also a problem since for example excessive IL-6 suppresses Th17 responses and reduces IL-17.
Then let’s not forget that the immune system uses reactive oxygen species (ROS) produced by phagocytes (neutrophils via NADPH oxidase) to kill Candida.
So a compromised immune system reduces ROS production, allowing Candida to survive where reduced host ROS enables Candida to maintain thiol-dependent enzymes, supporting metabolism, adhesion, and biofilm formation.
This is why some studies show that that CGD patients with impaired ROS production have a 5-fold higher risk of systemic candidiasis.
Moving on to antimicrobial peptides (AMPs) since they are quite connected with our immune system.
These, directly kill microbes by disrupting cell membranes, inducing reactive oxygen species (ROS), or inhibiting metabolic pathways, modulate immune responses by recruiting immune cells such as neutrophils and macrophages and stimulating cytokine production (IL-17 and IL-22 for example).
To put in perspective how important these are, histatin-5 reduced Candida albicans viability by 80% in vitro, and its absence in salivary deficiencies increased oral thrush risk by 4-fold.
Histatin-5 can even inhibit Candida’s metabolic enzymes such as ADH by up to 60%, disrupting energy production and nutrient acquisition.
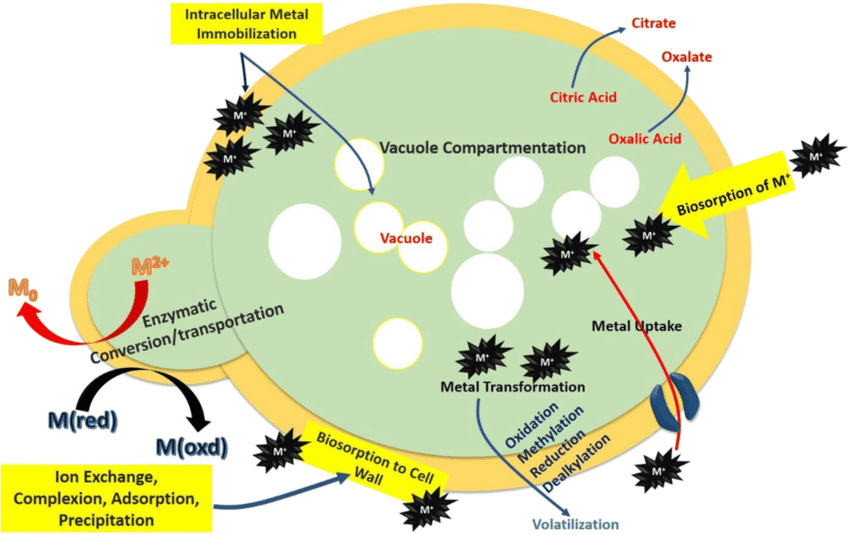
Now let’s talk about how hormonal issues such as low thyroid hormones and/or insulin resistance can affect a candida overgrowth.
First let’s discuss the impact of low thyroid hormones.
Thyroid hormones greatly regulate gut motility by influencing the enteric nervous system and smooth muscle contraction.
So low thyroid hormones or hypothyroidism, will slow peristalsis leading to low transit time and as it was previously stated, prolong the exposure to glucose and carbohydrates that fuels Candida’s glycolysis and fermentation, upregulating adhesins such as Als3 and quorum-sensing molecules such as tyrosol through the Ras1-cAMP-PKA pathway.
They also modulate immune responses by regulating T-cell differentiation, neutrophil activity and cytokine production so low T3/T4 levels reduce these functions leading to reduced phagocytosis by neutrophils and macrophages.
Hypothyroid patients for example are shown to have a 2-fold higher incidence of oral candidiasis due to reduced T-cell responses and sIgA.
But the problems don’t stop here.
Low thyroid hormones also lead to reduced antimicrobial peptide production since they upregulate AMPs like β-defensins and cathelicidins in epithelial cells.
One more problem is that hypothyroidism slows glucose metabolism, leading to elevated blood and tissue glucose levels and thus upregulates adhesins and quorum-sensing molecules once again enhancing adhesion sites and biofilms.
Now when it comes to insulin resistance, it first and foremost, leads to hyperglycemia, elevating glucose levels in blood, saliva, vaginal secretions and gut mucosa.
For example hyperglycemia in insulin-resistant patients increased vaginal Candida colonization by 3-fold, linked to elevated glycogen levels.
I also impairs immune responses by reducing neutrophil phagocytosis, macrophage activation, and cytokine production, suppresses AMP expression, slows gut motility due to autonomic neuropathy or inflammation and induces chronic low-grade inflammation via elevated proinflammatory cytokines.
When it comes to unbound iron, iron first and foremost is a critical cofactor for Candida’s metabolic enzymes, including those in glycolysis, the tricarboxylic acid (TCA) cycle and ergosterol biosynthesis.
How?
Well unbound iron is scavenged by Candida via siderophores such as ferrichrome or heme uptake systems such as Rbt5, fueling ATP production and growth.
It also activates the Ras1-cAMP-PKA pathway, upregulating adhesins and quorum-sensing molecules strengthening adhesion sites and biofilms.
Not only that but iron is also required for Candida’s yeast-to-hypha transition, a key virulence trait regulated by genes like EFG1 and Cph1.
It also supports Candida’s antioxidant enzymes, which neutralize ROS from host immune cells or antifungals while also being a cofactor for ERG11 (lanosterol 14-α-demethylase), a cytochrome P450 enzyme in the ergosterol biosynthesis pathway, so unbound iron also enhances ergosterol production.
There are of course more issues such as the fact that high iron levels reduce Lactobacillus populations by even 50%.
This is why you can find studies showing high iron levels increasing Candida albicans growth rates by 50%, iron supplementation increasing Candida biofilm formation by 3-fold, iron-rich environments increasing Candida hyphal formation by 50% .
Another important topic to discuss is how malnutrition and specific micronutrient deficiencies create an environment conducive to Candida albicans overgrowth.
Micronutrients like vitamin D, zinc, vitamin A, and vitamin C for example are critical for innate and adaptive immunity, including phagocytosis, cytokine production, and AMP expression.
Zinc and vitamin A deficiencies impair neutrophil and macrophage activity but also weaken mucosal epithelial barriers by reducing cell turnover and mucus production.
A zinc deficiency by itself is shown to reduce neutrophil phagocytosis by up to 50%.
Then, a vitamin D deficiency reduces IL-17 and IL-22 production by Th17 cells.
Then deficiencies in selenium, zinc, retinol, vitamin D and C are shown to reduce secretory IgA (sIgA).
A vitamin A deficiency for example reduces sIgA production by impairing B-cell differentiation and antibody secretion.
A zinc deficiency also disrupts sIgA synthesis by affecting B-cell activation and cytokine signaling.
A selenium deficiency may reduce sIgA levels by increasing oxidative stress and impairing B-cell function.
Of course, vitamin D and zinc are also essential for upregulating AMPs like β-defensins (hBD-2) and cathelicidins (LL-37) in epithelial cells.
Vitamin D deficiency reduced hBD-2 expression by 40% in mucosal tissues, increasing Candida colonization by 2-fold.
This can also be neglected when it comes to beneficial gut bacteria, yet a zinc deficiency can reduce Lactobacillus populations by 20%-50%.
Deficiencies in B vitamins and minerals such as magnesium can also lead to slow trasnit time (B vitamins affect neurotransmitters quite a lot (but also bile release) which is why so many people with general gut issues find B1, B6 (in the form of P5P) and B8 so helpful).
Not to even mention that deficiencies in B vitamins, magnesium and potassium impair glucose metabolism, exacerbating this effect.
And of course when it comes to high-carbohydrate, low-nutrient diets, can lead to relative hyperglycemia or poor glucose regulation, providing Candida with an abundant energy source.
When it comes to the mechanisms by which heavy metals promote a candida overgrowth we have the following.
First and foremost, they impair immune responses by reducing neutrophil and macrophage phagocytosis and suppressing cytokine production.
Candida can absorb methylmercury until it can be released in an organic form. Mercury also antagonizes selenium which is crucial for hypochlorite.
Heavy metals in general disrupt gut and mucosal microbiota by preferentially inhibiting beneficial bacteria, generate reactive oxygen species (ROS) in host cells, depleting host antioxidant defenses.
One more issue is that they can increase glucose availability by increasing inflamation and some also act as cofactors for ERG11, enhancing ergosterol production as well.
Now xenoestrogens, bind to estrogen receptors, upregulating Candida’s adhesin genes, they also increase glycogen availability in mucosal tissues and suppress immune responses mainly by reducing cytokine production.
Finally, when it comes to MCAS and histamine intolerance, they first one causes excessive mast cell degranulation, releasing histamine, pro-inflammatory cytokines such as IL-6 and TNF-α and prostaglandins.
Then, excessive histamine from MCAS and histamine intolerance increases mucosal permeability by stimulating H1 and H2 receptors, leading to epithelial damage and reduced mucus production.
They can also can slow gut motility through H2 receptor overstimulation.

First let’s discuss the impact of low thyroid hormones.
Thyroid hormones greatly regulate gut motility by influencing the enteric nervous system and smooth muscle contraction.
So low thyroid hormones or hypothyroidism, will slow peristalsis leading to low transit time and as it was previously stated, prolong the exposure to glucose and carbohydrates that fuels Candida’s glycolysis and fermentation, upregulating adhesins such as Als3 and quorum-sensing molecules such as tyrosol through the Ras1-cAMP-PKA pathway.
They also modulate immune responses by regulating T-cell differentiation, neutrophil activity and cytokine production so low T3/T4 levels reduce these functions leading to reduced phagocytosis by neutrophils and macrophages.
Hypothyroid patients for example are shown to have a 2-fold higher incidence of oral candidiasis due to reduced T-cell responses and sIgA.
But the problems don’t stop here.
Low thyroid hormones also lead to reduced antimicrobial peptide production since they upregulate AMPs like β-defensins and cathelicidins in epithelial cells.
One more problem is that hypothyroidism slows glucose metabolism, leading to elevated blood and tissue glucose levels and thus upregulates adhesins and quorum-sensing molecules once again enhancing adhesion sites and biofilms.
Now when it comes to insulin resistance, it first and foremost, leads to hyperglycemia, elevating glucose levels in blood, saliva, vaginal secretions and gut mucosa.
For example hyperglycemia in insulin-resistant patients increased vaginal Candida colonization by 3-fold, linked to elevated glycogen levels.
I also impairs immune responses by reducing neutrophil phagocytosis, macrophage activation, and cytokine production, suppresses AMP expression, slows gut motility due to autonomic neuropathy or inflammation and induces chronic low-grade inflammation via elevated proinflammatory cytokines.
When it comes to unbound iron, iron first and foremost is a critical cofactor for Candida’s metabolic enzymes, including those in glycolysis, the tricarboxylic acid (TCA) cycle and ergosterol biosynthesis.
How?
Well unbound iron is scavenged by Candida via siderophores such as ferrichrome or heme uptake systems such as Rbt5, fueling ATP production and growth.
It also activates the Ras1-cAMP-PKA pathway, upregulating adhesins and quorum-sensing molecules strengthening adhesion sites and biofilms.
Not only that but iron is also required for Candida’s yeast-to-hypha transition, a key virulence trait regulated by genes like EFG1 and Cph1.
It also supports Candida’s antioxidant enzymes, which neutralize ROS from host immune cells or antifungals while also being a cofactor for ERG11 (lanosterol 14-α-demethylase), a cytochrome P450 enzyme in the ergosterol biosynthesis pathway, so unbound iron also enhances ergosterol production.
There are of course more issues such as the fact that high iron levels reduce Lactobacillus populations by even 50%.
This is why you can find studies showing high iron levels increasing Candida albicans growth rates by 50%, iron supplementation increasing Candida biofilm formation by 3-fold, iron-rich environments increasing Candida hyphal formation by 50% .
Another important topic to discuss is how malnutrition and specific micronutrient deficiencies create an environment conducive to Candida albicans overgrowth.
Micronutrients like vitamin D, zinc, vitamin A, and vitamin C for example are critical for innate and adaptive immunity, including phagocytosis, cytokine production, and AMP expression.
Zinc and vitamin A deficiencies impair neutrophil and macrophage activity but also weaken mucosal epithelial barriers by reducing cell turnover and mucus production.
A zinc deficiency by itself is shown to reduce neutrophil phagocytosis by up to 50%.
Then, a vitamin D deficiency reduces IL-17 and IL-22 production by Th17 cells.
Then deficiencies in selenium, zinc, retinol, vitamin D and C are shown to reduce secretory IgA (sIgA).
A vitamin A deficiency for example reduces sIgA production by impairing B-cell differentiation and antibody secretion.
A zinc deficiency also disrupts sIgA synthesis by affecting B-cell activation and cytokine signaling.
A selenium deficiency may reduce sIgA levels by increasing oxidative stress and impairing B-cell function.
Of course, vitamin D and zinc are also essential for upregulating AMPs like β-defensins (hBD-2) and cathelicidins (LL-37) in epithelial cells.
Vitamin D deficiency reduced hBD-2 expression by 40% in mucosal tissues, increasing Candida colonization by 2-fold.
This can also be neglected when it comes to beneficial gut bacteria, yet a zinc deficiency can reduce Lactobacillus populations by 20%-50%.
Deficiencies in B vitamins and minerals such as magnesium can also lead to slow trasnit time (B vitamins affect neurotransmitters quite a lot (but also bile release) which is why so many people with general gut issues find B1, B6 (in the form of P5P) and B8 so helpful).
Not to even mention that deficiencies in B vitamins, magnesium and potassium impair glucose metabolism, exacerbating this effect.
And of course when it comes to high-carbohydrate, low-nutrient diets, can lead to relative hyperglycemia or poor glucose regulation, providing Candida with an abundant energy source.
When it comes to the mechanisms by which heavy metals promote a candida overgrowth we have the following.
First and foremost, they impair immune responses by reducing neutrophil and macrophage phagocytosis and suppressing cytokine production.
Candida can absorb methylmercury until it can be released in an organic form. Mercury also antagonizes selenium which is crucial for hypochlorite.
Heavy metals in general disrupt gut and mucosal microbiota by preferentially inhibiting beneficial bacteria, generate reactive oxygen species (ROS) in host cells, depleting host antioxidant defenses.
One more issue is that they can increase glucose availability by increasing inflamation and some also act as cofactors for ERG11, enhancing ergosterol production as well.
Now xenoestrogens, bind to estrogen receptors, upregulating Candida’s adhesin genes, they also increase glycogen availability in mucosal tissues and suppress immune responses mainly by reducing cytokine production.
Finally, when it comes to MCAS and histamine intolerance, they first one causes excessive mast cell degranulation, releasing histamine, pro-inflammatory cytokines such as IL-6 and TNF-α and prostaglandins.
Then, excessive histamine from MCAS and histamine intolerance increases mucosal permeability by stimulating H1 and H2 receptors, leading to epithelial damage and reduced mucus production.
They can also can slow gut motility through H2 receptor overstimulation.
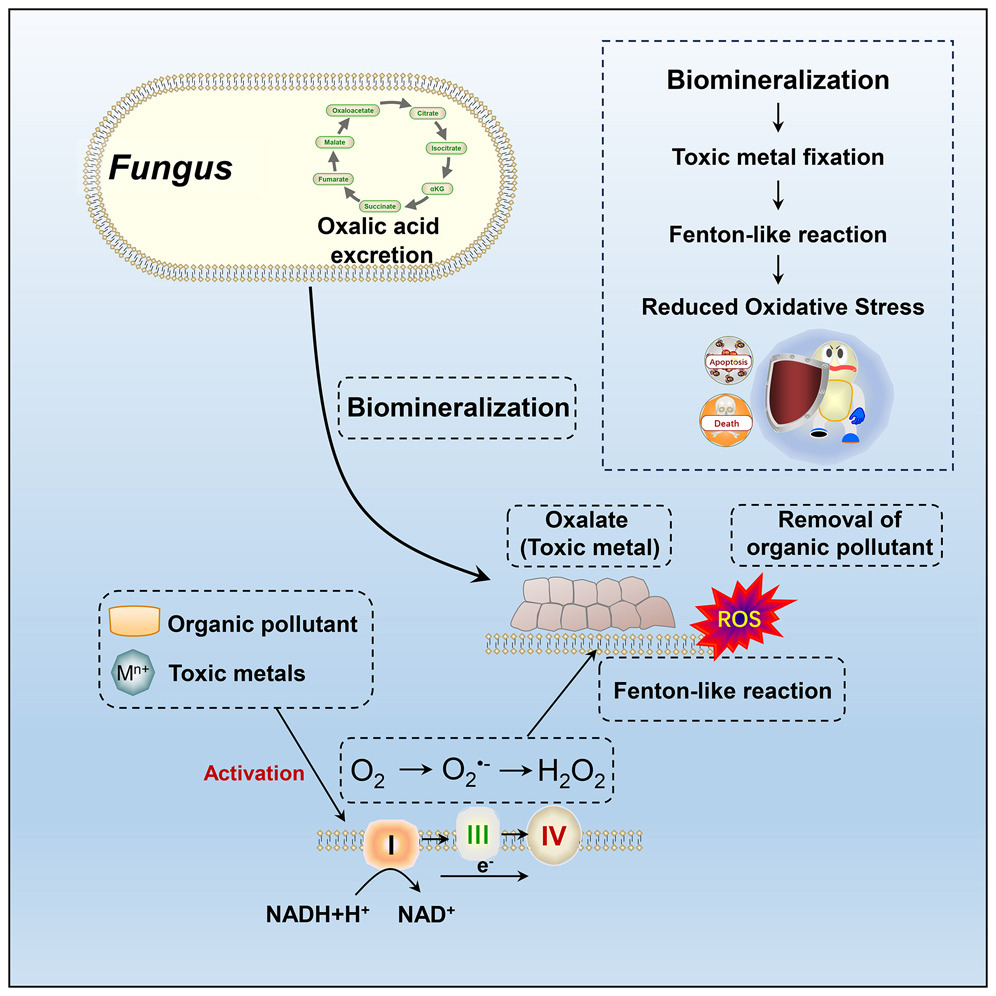
Now i am fully aware that these may sound too complicated so here are some basic suggestions.
Number 1: First DO NO HARM.
In case for example of oral or toe infections, topicals SHOULD be used while other principles such as the following are applied.
Some of these that target a bit of everything include:
-Avoiding the following foods and dietary habits that can negatively impact gut health:
1. The consumption of highly processed foods.
These are problematic for a variety of reasons.
Most of them for example will have emulsifiers and preservatives in them which are shown to disrupt the gut microbiome by altering bacterial composition and increasing inflammation.
Then most will be devoided of nutrients and the ones that aren’t often are high in histamine and also packaged in ways that leads to the leakage of toxins such ass aluminum (thik canned seafood for example).
2. The consumption of too many acellular carbs (think refined sugars).
These in excess promote the growth of harmful bacteria and yeast, leading to dysbiosis.
They also increase gut permeability by disrupting tight junction proteins.
3. Artificial sweeteners such as aspartame, sucralose, saccharin and acesulfame potassium.
Even though it is in fact true that in order for these to show a direct and immediate impact on things such as cancer, the doses that are required are quite high, in the context of gut health, even in small amounts they can alter gut bacterial diversity, reducing beneficial species like Bifidobacterium and increasing pro-inflammatory bacteria.
4. High O6 and trans fats.
Trans and easily oxidized fats produce pr inflammatory cytokines, damaging the gut lining and are also shown to reduce beneficial bacteria.
5. Excessive alcohol intake (if you have any gut issue, take a 2 month break from all alcoholic beverages, and if you have any pathogen overgrowth avoid all beers, ciders and wines for 4 months).
Alcohol is known to disrupt tight junctions, reduce microbial diversity and in the case of detoxing, it will of course over burden the liver.
6. Hybridized grains.
Overall, after 1960 we started creating wheat hybrids with the purpose of the plant being able to survive some pretty harsh climates.
Now the gluten that’s presented in hybridized grains is so hard to digest that it will inevitably lead to your gut taking such as big hit if they are consumed very often.
Note: Gluten is a mixture of different protein types (mainly gliadin and glutenin) or a protein composite that is found in things such as wheat, rye and barley.
Not only that but please also consider that we no longer sprout or ferment the grains a lot of the times which is designed to break down some of the gluten but also compounds such as phytic acid.
Then in a lot of countries such as the states, grains are probably the number 2 (soy is probably the number 1) thing that is sprayed with enormous amounts of herbicides such as glyphosate.
Gluten also triggers the release of zonulin (a protein that regulates the permeability of the intestinal wall by modulating the tight junctions).
Then, we have the issue of mycotoxin contamination and of course the fact that plenty of grains are fortified with folic acid and forms of iron that are basically iron shavings.
Some people are also sensitive to plant albumin.
7. A1 dairy and the overconsumption of dairy overall (even too much milk can dilute stomach acid for example and cause issues).
A2 dairy have proline in the 67 position of the beta casein chain while A1 dairy have histidine.
This can make a night and day difference when consuming dairy and makes A1 dairy a no go for people struggling with mast cell issues and and will inevitably lead to inflammation compared to A2.
8. The over consumption of high-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) foods such as onions, garlic, beans, lentils, wheat, apples, pears, stone fruits, honey, high-fructose fruits etc that lead to fast fermentation by gut bacteria.
9. Foods rich in phytates and oxalates that will bind to minerals such as zinc, iron, and calcium, reducing their absorption and potentially impairing gut cell function.
10. The overconsumption of caffeine (>100mg/day) increases peristalsis and reduces mucus production.
11. Herbicides and pesticides like glyphosate that act as antibiotics and reduce beneficial gut bacteria.
Note: N-phosphomethylglycine aka glyphosate, works by inhibiting the enzyme 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase.
This results in disrupting the shikimic acid pathway and reducing the aromatic amino acids.
In general, the shikimic acid pathway is responsible for the production of vitamins and aromatic amino acids such as phenylalanine through EPSP synthase so disrupting it negatively affects eNOS, the pancreas, adrenals, SOD, CAT and GPx enzymes, glutamate, GABA, the metabolism of retinoic acid and inhibiting GGT plus G6PD and can even exacerbate the damage that aluminum causes.
12. Irregular eating patterns that reduce gastric acid and enzyme production and disrupt the gut’s circadian rhythm, microbial balance and motility.
13. Eating too quickly or large portion sizes both of which will lead to incomplete digestion and fermentation in the gut.
14. Eating late at night will also disrupt the gut’s circadian rhythm, impairing microbial balance and motility. It will also increase the risk of acid reflux, as lying down after eating allows stomach acid to enter the esophagus.
15. Inadequate hydration which was previously discussed and will reduce mucus production while also slowing motility.
16. Frequent snacking.
Eating something every 1-2 hours, does not allow the migrating motor complex (MMC (a cyclical pattern of peristaltic activity that occurs during fasting state)) to clear bacteria from the small intestine for example.
Number 1: First DO NO HARM.
In case for example of oral or toe infections, topicals SHOULD be used while other principles such as the following are applied.
Some of these that target a bit of everything include:
-Avoiding the following foods and dietary habits that can negatively impact gut health:
1. The consumption of highly processed foods.
These are problematic for a variety of reasons.
Most of them for example will have emulsifiers and preservatives in them which are shown to disrupt the gut microbiome by altering bacterial composition and increasing inflammation.
Then most will be devoided of nutrients and the ones that aren’t often are high in histamine and also packaged in ways that leads to the leakage of toxins such ass aluminum (thik canned seafood for example).
2. The consumption of too many acellular carbs (think refined sugars).
These in excess promote the growth of harmful bacteria and yeast, leading to dysbiosis.
They also increase gut permeability by disrupting tight junction proteins.
3. Artificial sweeteners such as aspartame, sucralose, saccharin and acesulfame potassium.
Even though it is in fact true that in order for these to show a direct and immediate impact on things such as cancer, the doses that are required are quite high, in the context of gut health, even in small amounts they can alter gut bacterial diversity, reducing beneficial species like Bifidobacterium and increasing pro-inflammatory bacteria.
4. High O6 and trans fats.
Trans and easily oxidized fats produce pr inflammatory cytokines, damaging the gut lining and are also shown to reduce beneficial bacteria.
5. Excessive alcohol intake (if you have any gut issue, take a 2 month break from all alcoholic beverages, and if you have any pathogen overgrowth avoid all beers, ciders and wines for 4 months).
Alcohol is known to disrupt tight junctions, reduce microbial diversity and in the case of detoxing, it will of course over burden the liver.
6. Hybridized grains.
Overall, after 1960 we started creating wheat hybrids with the purpose of the plant being able to survive some pretty harsh climates.
Now the gluten that’s presented in hybridized grains is so hard to digest that it will inevitably lead to your gut taking such as big hit if they are consumed very often.
Note: Gluten is a mixture of different protein types (mainly gliadin and glutenin) or a protein composite that is found in things such as wheat, rye and barley.
Not only that but please also consider that we no longer sprout or ferment the grains a lot of the times which is designed to break down some of the gluten but also compounds such as phytic acid.
Then in a lot of countries such as the states, grains are probably the number 2 (soy is probably the number 1) thing that is sprayed with enormous amounts of herbicides such as glyphosate.
Gluten also triggers the release of zonulin (a protein that regulates the permeability of the intestinal wall by modulating the tight junctions).
Then, we have the issue of mycotoxin contamination and of course the fact that plenty of grains are fortified with folic acid and forms of iron that are basically iron shavings.
Some people are also sensitive to plant albumin.
7. A1 dairy and the overconsumption of dairy overall (even too much milk can dilute stomach acid for example and cause issues).
A2 dairy have proline in the 67 position of the beta casein chain while A1 dairy have histidine.
This can make a night and day difference when consuming dairy and makes A1 dairy a no go for people struggling with mast cell issues and and will inevitably lead to inflammation compared to A2.
8. The over consumption of high-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) foods such as onions, garlic, beans, lentils, wheat, apples, pears, stone fruits, honey, high-fructose fruits etc that lead to fast fermentation by gut bacteria.
9. Foods rich in phytates and oxalates that will bind to minerals such as zinc, iron, and calcium, reducing their absorption and potentially impairing gut cell function.
10. The overconsumption of caffeine (>100mg/day) increases peristalsis and reduces mucus production.
11. Herbicides and pesticides like glyphosate that act as antibiotics and reduce beneficial gut bacteria.
Note: N-phosphomethylglycine aka glyphosate, works by inhibiting the enzyme 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase.
This results in disrupting the shikimic acid pathway and reducing the aromatic amino acids.
In general, the shikimic acid pathway is responsible for the production of vitamins and aromatic amino acids such as phenylalanine through EPSP synthase so disrupting it negatively affects eNOS, the pancreas, adrenals, SOD, CAT and GPx enzymes, glutamate, GABA, the metabolism of retinoic acid and inhibiting GGT plus G6PD and can even exacerbate the damage that aluminum causes.
12. Irregular eating patterns that reduce gastric acid and enzyme production and disrupt the gut’s circadian rhythm, microbial balance and motility.
13. Eating too quickly or large portion sizes both of which will lead to incomplete digestion and fermentation in the gut.
14. Eating late at night will also disrupt the gut’s circadian rhythm, impairing microbial balance and motility. It will also increase the risk of acid reflux, as lying down after eating allows stomach acid to enter the esophagus.
15. Inadequate hydration which was previously discussed and will reduce mucus production while also slowing motility.
16. Frequent snacking.
Eating something every 1-2 hours, does not allow the migrating motor complex (MMC (a cyclical pattern of peristaltic activity that occurs during fasting state)) to clear bacteria from the small intestine for example.
Number 2: Rebuild the natural protection mechanisms with SAFE tools.
-GET SUN.
Melanin fights Candida directly, while vitamin D upregulates LL-37 (an antimicrobial peptide), acts as an immune modulator by binding to VDRs in immune cells, such as T-cells, B-cells, and macrophages, within the gut-associated lymphoid tissue (GALT).
-SWEAT and do the big 6.
Very basic ways to manage toxin overload without many side effects.
-MOVE.
"MIND BLOWING INFORMATION GEORGE".
Be quiet and don't take this for granted.
Regular movement is crucial for our gut health/
Movement:
-Stimulates the smooth muscles of the GI tract, promoting peristalsis.
-Enhances HCl, enzyme, and bile production for digestion.
-Promotes MMC activity.
-Supports lymphatic flow.
-Boosts antimicrobial peptide production and reduces inflammation.
-Influences the diversity and composition of the gut microbiota. Physical activity for example has shown to increase the production of short-chain fatty acids (SCFAs) like butyrate and also promote the growth of beneficial bacterial species, such as Lactobacillus and Bifidobacterium.
-Enhances circulation, ensuring that the gut receives adequate oxygen and nutrients to support its high metabolic demands.
-Regular movement has anti-inflammatory effects that protect the gut lining and prevent chronic inflammation, a key driver of gut disorders.
Name one pill that does all these (you can't).
-Provide KEY nutrients such as zinc, B vitamins, potassium, magnesium, selenium, vitamin C, E and glutamine.
Zinc for example is a cofactor for enzymes involved in gastric acid secretion in the stomach, competes for binding sites on gut epithelial cells with pathogens and strengthens the intestinal lining by supporting tight junction proteins while also being critical for the gut-associated lymphoid tissue (GALT) and the immune system.
While selenium, is a cofactor for selenoproteins, such as glutathione peroxidase, which protect gut cells from oxidative damage and support immune cell function (it supports T-cell proliferation and cytokine production for example).
Vitamin E also protects gut cell membranes from lipid peroxidation and ROS overall.
-GET SUN.
Melanin fights Candida directly, while vitamin D upregulates LL-37 (an antimicrobial peptide), acts as an immune modulator by binding to VDRs in immune cells, such as T-cells, B-cells, and macrophages, within the gut-associated lymphoid tissue (GALT).
-SWEAT and do the big 6.
Very basic ways to manage toxin overload without many side effects.
-MOVE.
"MIND BLOWING INFORMATION GEORGE".
Be quiet and don't take this for granted.
Regular movement is crucial for our gut health/
Movement:
-Stimulates the smooth muscles of the GI tract, promoting peristalsis.
-Enhances HCl, enzyme, and bile production for digestion.
-Promotes MMC activity.
-Supports lymphatic flow.
-Boosts antimicrobial peptide production and reduces inflammation.
-Influences the diversity and composition of the gut microbiota. Physical activity for example has shown to increase the production of short-chain fatty acids (SCFAs) like butyrate and also promote the growth of beneficial bacterial species, such as Lactobacillus and Bifidobacterium.
-Enhances circulation, ensuring that the gut receives adequate oxygen and nutrients to support its high metabolic demands.
-Regular movement has anti-inflammatory effects that protect the gut lining and prevent chronic inflammation, a key driver of gut disorders.
Name one pill that does all these (you can't).
-Provide KEY nutrients such as zinc, B vitamins, potassium, magnesium, selenium, vitamin C, E and glutamine.
Zinc for example is a cofactor for enzymes involved in gastric acid secretion in the stomach, competes for binding sites on gut epithelial cells with pathogens and strengthens the intestinal lining by supporting tight junction proteins while also being critical for the gut-associated lymphoid tissue (GALT) and the immune system.
While selenium, is a cofactor for selenoproteins, such as glutathione peroxidase, which protect gut cells from oxidative damage and support immune cell function (it supports T-cell proliferation and cytokine production for example).
Vitamin E also protects gut cell membranes from lipid peroxidation and ROS overall.
Work on bile:
https://x.com/Helios_Movement/status/1884202740382888191
-GET ON THAT S. Boulardii
pubmed.ncbi.nlm.nih.gov/21077734/
-Consider safe prokinetics such as:
-Ginger.
-Dandelion root.
-Artichoke leaf.
-Peppermint.
-Licorice root.
-Go to sleep and fix your CR.
When it comes to circadian health and sleep:
-The gut microbiota dynamics follow a 24-hour cycle (the entire G.I tract follows a circadian pattern, bacterial populations, gastric emptying, peristalsis, and enzyme secretion peak at specific times to aid digestion and shift work for example has been shown to alter microbial composition, reducing beneficial bacteria and promoting harmful species).
Note: This is why, believe it or not, the simple act of eating at consistent times has improved the gut health of so many people.
-Melatonin acts as a potent antioxidant in the gastrointestinal tract, enhances mucosal repair and regulates gut motility.
-Sleep is critical for cellular repair and tissue regeneration (through growth hormone for example) throughout the body and thus the gastrointestinal tract as well.
-During sleep, immune cells like T-cells and B-cells in the gut are replenished and primed to combat pathogens
-Then during sleep, anti-inflammatory cytokines like IL-10 are upregulated.
-Also, poor sleep or circadian disruption elevate cortisol, which reduce microbial diversity, increase gut permeability, and also promote inflammation.
-Then the gut-associated lymphoid tissue (GALT) also relies on circadian rhythms to maintain its surveillance and response capabilities.
-During sleep, immune cells like T-cells and B-cells in the gut are replenished and primed to combat pathogens.
-Adequate sleep enhances the production of immunoglobulin A (IgA), an antibody that coats the gut mucosa to neutralize harmful bacteria and viruses.
-Supports the migrating motor complex (MMC), which clears bacteria from the small intestine during fasting.
Number 3: Focus on tight junction health since without this, antifungals and biofilms disruptors can make the condition worse.
Tight junctions (TJs) are protein-based structures (their structure is broken down in a moment) that form a selective barrier between epithelial (the top region of adjacent epithelial cells) and endothelial cells primarily in the gastrointestinal (GI) tract which makes them components of the intestinal mucosal barrier.
Without TJs enterocytes and colonocytes can not function properly and overall, tight junctions are essential for preventing the uncontrolled passage of things such as bacteria, toxins and undigested food into the bloodstream maintaining a selective barrier (they allow regulated paracellular transport of small molecules such as ions, water and glucose for example through claudin pores).
They also assist the gut-associated lymphoid tissue (GALT), limit translocation of lipopolysaccharides (LPS), facilitate mucus layer integrity and do much more which is why increased permeability or leaky gut, leads to system inflammation (TLR4 is activated and cytokines such as IL-6 and TNF-a are activated after LPS, food antigens or toxins enter circulation for example), contributes to to food sensitivities (IgG-mediated), autoimmune disorder and of course, issues such as pathogen overgrowths, IBS and IBD where for example even up to 80% of people with IBS have increased permeability.
Now TJs are made out of:
1. Transmembrane proteins such as:
-Claudins which is a family of 27 proteins that determine TJ permeability (claudin-1 and -3 for example form tight seals, while claudin-2 increases pore size).
-Occludin that enhances TJ stability and regulates paracellular transport by organising TJ strands and linking them to the actin cytoskeleton via ZO protein for example or by regulating paracellular transport of ions and small molecules.
-JAMs that modulate TJ assembly and immune cell transmigration.
For example, they contribute to the initial formation of TJs by recruiting scaffolding proteins and facilitating cell-cell adhesion.
They also interact with claudins and occludin while also regulating leukocyte and monocyte transmigration across endothelial and epithelial barriers.
-Tricellulin that strengthens junctions at tricellular contacts (where three cells meet).
For example, it forms a barrier to prevent paracellular flux of macromolecules and pathogens at these sites while also binding to LSR (lipolysis-stimulated lipoprotein receptor) to maintain tricellular integrity.
2. Intracellular scaffolding proteins such as:
-Zonula occludens (ZO) proteins are members of the membrane-associated guanylate kinase (MAGUK) family and serve as intracellular scaffolding proteins that place TJ transmembrane proteins (claudins, occludin, JAMs, tricellulin) to the actin cytoskeleton, stabilize TJ structure and mediate signaling.
-Associated (cytoskeletal) proteins actin and myosin filaments in the cytoskeleton dynamically regulate TJ opening/closing via phosphorylation responding to mechanical and biochemical cues.
Now here's how you can improve the function of tight junctions in a nutshell:
Number 1: If you have any inflammatory condition such as MCAS you need to take it into consideration.
Number 2: Eliminate gluten, sugar, alcohol, the overconsumption of high FODMAP foods, NSAIDs, PPIs, heavy metals and glyphosate.
Number 3: Relax.
As stated, cortisol disrupts TJs by dysregulating the HPA axis and it also activates MLCK.
Number 4: Go to sleep and get sun.
Poor sleep and CR disrupt TJ protein expression while sunlight and vitamin D upregulates it.
Number 5: Provide enough zinc (stabilizes TJs), L glutamine (enhances TJ protein expression), retinol and polyphenols.
Number 6: Pick the following probiotic that works best for you:
-Lactobacillus rhamnosus GG.
-Bifidobacterium longum.
-Saccharomyces boulardii.
If these do not work for you or you can't use them, consider:
-Quercetin
-Curcumin
NAC can also help in the specific context of people with Chrohn's
-DGL
pubmed.ncbi.nlm.nih.gov/21077734/
-Consider safe prokinetics such as:
-Ginger.
-Dandelion root.
-Artichoke leaf.
-Peppermint.
-Licorice root.
-Go to sleep and fix your CR.
When it comes to circadian health and sleep:
-The gut microbiota dynamics follow a 24-hour cycle (the entire G.I tract follows a circadian pattern, bacterial populations, gastric emptying, peristalsis, and enzyme secretion peak at specific times to aid digestion and shift work for example has been shown to alter microbial composition, reducing beneficial bacteria and promoting harmful species).
Note: This is why, believe it or not, the simple act of eating at consistent times has improved the gut health of so many people.
-Melatonin acts as a potent antioxidant in the gastrointestinal tract, enhances mucosal repair and regulates gut motility.
-Sleep is critical for cellular repair and tissue regeneration (through growth hormone for example) throughout the body and thus the gastrointestinal tract as well.
-During sleep, immune cells like T-cells and B-cells in the gut are replenished and primed to combat pathogens
-Then during sleep, anti-inflammatory cytokines like IL-10 are upregulated.
-Also, poor sleep or circadian disruption elevate cortisol, which reduce microbial diversity, increase gut permeability, and also promote inflammation.
-Then the gut-associated lymphoid tissue (GALT) also relies on circadian rhythms to maintain its surveillance and response capabilities.
-During sleep, immune cells like T-cells and B-cells in the gut are replenished and primed to combat pathogens.
-Adequate sleep enhances the production of immunoglobulin A (IgA), an antibody that coats the gut mucosa to neutralize harmful bacteria and viruses.
-Supports the migrating motor complex (MMC), which clears bacteria from the small intestine during fasting.
Number 3: Focus on tight junction health since without this, antifungals and biofilms disruptors can make the condition worse.
Tight junctions (TJs) are protein-based structures (their structure is broken down in a moment) that form a selective barrier between epithelial (the top region of adjacent epithelial cells) and endothelial cells primarily in the gastrointestinal (GI) tract which makes them components of the intestinal mucosal barrier.
Without TJs enterocytes and colonocytes can not function properly and overall, tight junctions are essential for preventing the uncontrolled passage of things such as bacteria, toxins and undigested food into the bloodstream maintaining a selective barrier (they allow regulated paracellular transport of small molecules such as ions, water and glucose for example through claudin pores).
They also assist the gut-associated lymphoid tissue (GALT), limit translocation of lipopolysaccharides (LPS), facilitate mucus layer integrity and do much more which is why increased permeability or leaky gut, leads to system inflammation (TLR4 is activated and cytokines such as IL-6 and TNF-a are activated after LPS, food antigens or toxins enter circulation for example), contributes to to food sensitivities (IgG-mediated), autoimmune disorder and of course, issues such as pathogen overgrowths, IBS and IBD where for example even up to 80% of people with IBS have increased permeability.
Now TJs are made out of:
1. Transmembrane proteins such as:
-Claudins which is a family of 27 proteins that determine TJ permeability (claudin-1 and -3 for example form tight seals, while claudin-2 increases pore size).
-Occludin that enhances TJ stability and regulates paracellular transport by organising TJ strands and linking them to the actin cytoskeleton via ZO protein for example or by regulating paracellular transport of ions and small molecules.
-JAMs that modulate TJ assembly and immune cell transmigration.
For example, they contribute to the initial formation of TJs by recruiting scaffolding proteins and facilitating cell-cell adhesion.
They also interact with claudins and occludin while also regulating leukocyte and monocyte transmigration across endothelial and epithelial barriers.
-Tricellulin that strengthens junctions at tricellular contacts (where three cells meet).
For example, it forms a barrier to prevent paracellular flux of macromolecules and pathogens at these sites while also binding to LSR (lipolysis-stimulated lipoprotein receptor) to maintain tricellular integrity.
2. Intracellular scaffolding proteins such as:
-Zonula occludens (ZO) proteins are members of the membrane-associated guanylate kinase (MAGUK) family and serve as intracellular scaffolding proteins that place TJ transmembrane proteins (claudins, occludin, JAMs, tricellulin) to the actin cytoskeleton, stabilize TJ structure and mediate signaling.
-Associated (cytoskeletal) proteins actin and myosin filaments in the cytoskeleton dynamically regulate TJ opening/closing via phosphorylation responding to mechanical and biochemical cues.
Now here's how you can improve the function of tight junctions in a nutshell:
Number 1: If you have any inflammatory condition such as MCAS you need to take it into consideration.
Number 2: Eliminate gluten, sugar, alcohol, the overconsumption of high FODMAP foods, NSAIDs, PPIs, heavy metals and glyphosate.
Number 3: Relax.
As stated, cortisol disrupts TJs by dysregulating the HPA axis and it also activates MLCK.
Number 4: Go to sleep and get sun.
Poor sleep and CR disrupt TJ protein expression while sunlight and vitamin D upregulates it.
Number 5: Provide enough zinc (stabilizes TJs), L glutamine (enhances TJ protein expression), retinol and polyphenols.
Number 6: Pick the following probiotic that works best for you:
-Lactobacillus rhamnosus GG.
-Bifidobacterium longum.
-Saccharomyces boulardii.
If these do not work for you or you can't use them, consider:
-Quercetin
-Curcumin
NAC can also help in the specific context of people with Chrohn's
-DGL

Number 4: Start working on biofilms.
Biofilms are organized “groups” of microorganisms such as bacteria, fungi, archaea, or protozoa commonly formed in the gut, oral cavity and sinuses.
They are embedded in a self-produced extracellular matrix composed of polysaccharides, proteins, and extracellular DNA (eDNA) that acts as a physical barrier and makes them highly resistant to antibiotics and antifungals.
Biofilm formation starts with planktonic microbes adhering to a surface through something called van der Waals forces or specific adhesins, then microbes start producing matrix components. T
hen the maturation process begins where cell division and matrix expansion form microcolonies starts and finally three-dimensional structures develop, with channels for nutrient flow.
This process is regulated by quorum sensing which is a cell-to-cell communication system using signaling molecules to coordinate gene expression, enhancing virulence and resistance.
The three biofilm disruptors with the least chances of backfiring include:
1. Apolactoferrin
Lactoferrin comes in two forms:
-Native lactoferrin
-Apolactoferrin
Both can be quite valuable but the first one is iron bound and the second one is not which allows it to deprive pathogens of iron and thus helping us actually starve iron-dependent pathogens like candida albicans.
It also prevents pathogens from adhering to surfaces by interfering with adhesins and inhibits quorum sensing.
It also seems to be better tolerated from people who experience discomfort from things such as NAC and bromelain.
And it can of course help people who have hereditary hemochromatosis and find that even though flavonoids help their markers and some symptoms, they lower their libido too much.
2. L. reuteri DSM 17938, ATCC PTA 5289.
These are suggested since they produce reuterin that has broad-spectrum antimicrobial properties that can also disrupt EPS, competes for adhesion sites and inhibits quorum-sensing pathways.
3. Curcumin.
This one inhibits quorum sensing and disrupts bacterial cell membranes as well.
Other ones that can help include: NAC, berberine, garlic extract, bromelain and serrapeptase
Number 5: Nuke, bind and manage die offs.
This is where antifungals such as carplilic acid, sodium caprylate, molybdenum, berberine, ceylon cinnamon, blackseed oil paired with prokinetics such as ginger and binder such as activated charcoal come into play.
Disclaimer: If you do everything else right, you really should feel die off with small amounts of these.
If not (and i've seen this happen) the tips that were addressed so far might actually have already taken care of the overgrowth so consider taking the tests again.
When it comes to die offs, go and read this:
x.com/Helios_Movemen…
Number 6: Re-storing the balance.
This one will require testing in order to see if the overgrowth is at bay and tools such as colostrum, zinc, B2 and B1 can be used.
Biofilms are organized “groups” of microorganisms such as bacteria, fungi, archaea, or protozoa commonly formed in the gut, oral cavity and sinuses.
They are embedded in a self-produced extracellular matrix composed of polysaccharides, proteins, and extracellular DNA (eDNA) that acts as a physical barrier and makes them highly resistant to antibiotics and antifungals.
Biofilm formation starts with planktonic microbes adhering to a surface through something called van der Waals forces or specific adhesins, then microbes start producing matrix components. T
hen the maturation process begins where cell division and matrix expansion form microcolonies starts and finally three-dimensional structures develop, with channels for nutrient flow.
This process is regulated by quorum sensing which is a cell-to-cell communication system using signaling molecules to coordinate gene expression, enhancing virulence and resistance.
The three biofilm disruptors with the least chances of backfiring include:
1. Apolactoferrin
Lactoferrin comes in two forms:
-Native lactoferrin
-Apolactoferrin
Both can be quite valuable but the first one is iron bound and the second one is not which allows it to deprive pathogens of iron and thus helping us actually starve iron-dependent pathogens like candida albicans.
It also prevents pathogens from adhering to surfaces by interfering with adhesins and inhibits quorum sensing.
It also seems to be better tolerated from people who experience discomfort from things such as NAC and bromelain.
And it can of course help people who have hereditary hemochromatosis and find that even though flavonoids help their markers and some symptoms, they lower their libido too much.
2. L. reuteri DSM 17938, ATCC PTA 5289.
These are suggested since they produce reuterin that has broad-spectrum antimicrobial properties that can also disrupt EPS, competes for adhesion sites and inhibits quorum-sensing pathways.
3. Curcumin.
This one inhibits quorum sensing and disrupts bacterial cell membranes as well.
Other ones that can help include: NAC, berberine, garlic extract, bromelain and serrapeptase
Number 5: Nuke, bind and manage die offs.
This is where antifungals such as carplilic acid, sodium caprylate, molybdenum, berberine, ceylon cinnamon, blackseed oil paired with prokinetics such as ginger and binder such as activated charcoal come into play.
Disclaimer: If you do everything else right, you really should feel die off with small amounts of these.
If not (and i've seen this happen) the tips that were addressed so far might actually have already taken care of the overgrowth so consider taking the tests again.
When it comes to die offs, go and read this:
x.com/Helios_Movemen…
Number 6: Re-storing the balance.
This one will require testing in order to see if the overgrowth is at bay and tools such as colostrum, zinc, B2 and B1 can be used.
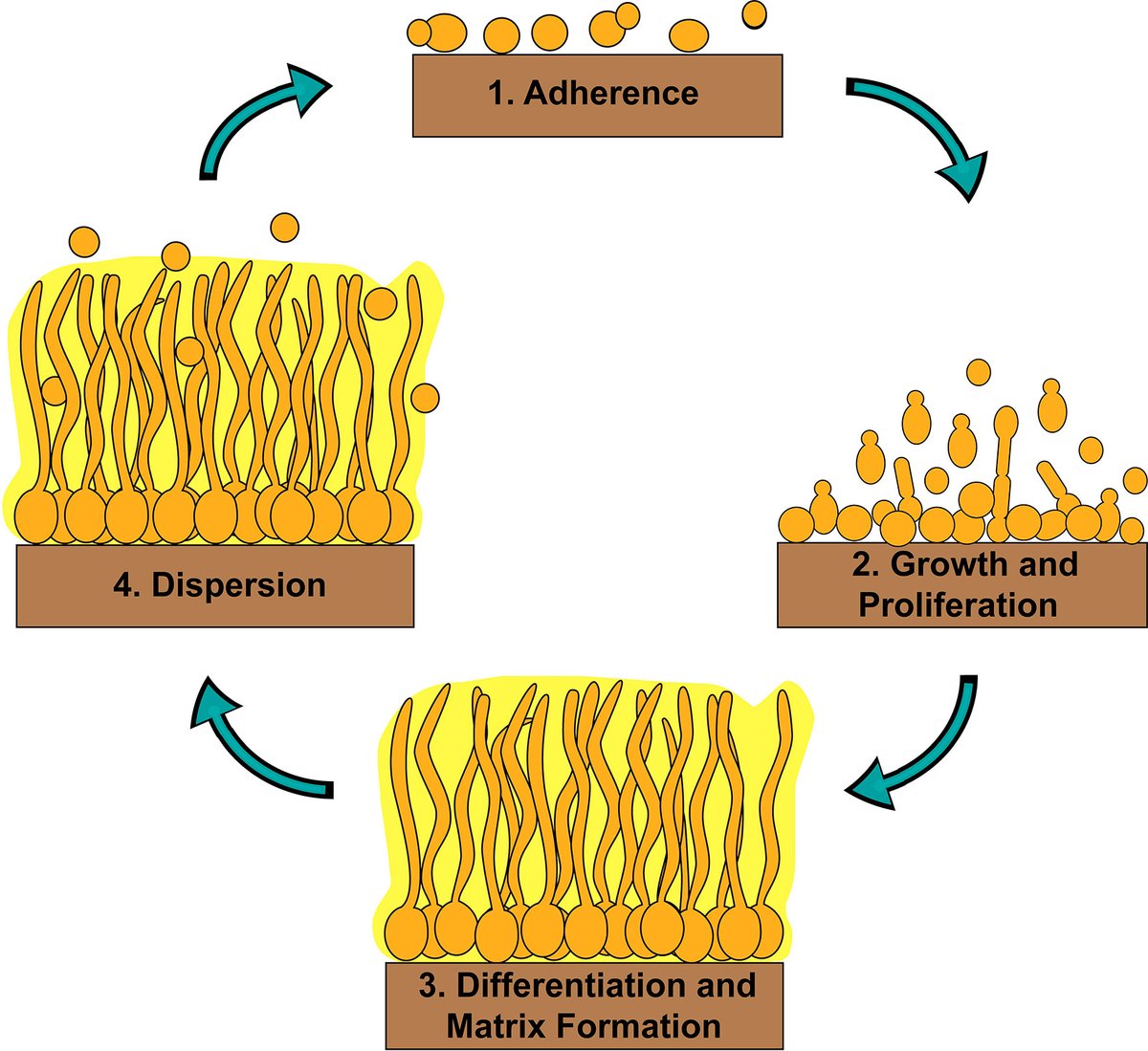
That's all.
I hope that you learned something from this thread.
If you did, make sure to leave a like/RT.
I hope that you learned something from this thread.
If you did, make sure to leave a like/RT.
https://x.com/Helios_Movement/status/1951646590839493009
For more on a variety of health topics whether that's called sleep, hormones, gut health, skin health and so on, go here:
fitandball.gumroad.com/l/georgesystem…
fitandball.gumroad.com/l/georgesystem…
Some studies you can look into:
link.springer.com/article/10.100…
pubmed.ncbi.nlm.nih.gov/22955356/
pubmed.ncbi.nlm.nih.gov/38779729/
onlinelibrary.wiley.com/doi/full/10.10…
sciencedirect.com/science/articl….
pmc.ncbi.nlm.nih.gov/articles/PMC74…
pmc.ncbi.nlm.nih.gov/articles/PMC11…
pmc.ncbi.nlm.nih.gov/articles/PMC77…
pmc.ncbi.nlm.nih.gov/articles/PMC10…
link.springer.com/article/10.100…
pubmed.ncbi.nlm.nih.gov/22955356/
pubmed.ncbi.nlm.nih.gov/38779729/
onlinelibrary.wiley.com/doi/full/10.10…
sciencedirect.com/science/articl….
pmc.ncbi.nlm.nih.gov/articles/PMC74…
pmc.ncbi.nlm.nih.gov/articles/PMC11…
pmc.ncbi.nlm.nih.gov/articles/PMC77…
pmc.ncbi.nlm.nih.gov/articles/PMC10…
mdpi.com/1422-0067/23/1…
sciencedirect.com/science/articl…
pmc.ncbi.nlm.nih.gov/articles/PMC90…
mdpi.com/2504-3900/61/1…
jmb.or.kr/journal/view.h…
frontiersin.org/journals/cellu…
pmc.ncbi.nlm.nih.gov/articles/PMC75…
pmc.ncbi.nlm.nih.gov/articles/PMC10…
sciencedirect.com/science/articl…
pmc.ncbi.nlm.nih.gov/articles/PMC90…
mdpi.com/2504-3900/61/1…
jmb.or.kr/journal/view.h…
frontiersin.org/journals/cellu…
pmc.ncbi.nlm.nih.gov/articles/PMC75…
pmc.ncbi.nlm.nih.gov/articles/PMC10…
journals.asm.org/doi/10.1128/sp…
pmc.ncbi.nlm.nih.gov/articles/PMC11…
pmc.ncbi.nlm.nih.gov/articles/PMC68…
researchgate.net/publication/31…
researchgate.net/publication/29…
researchgate.net/publication/24…
pmc.ncbi.nlm.nih.gov/articles/PMC10…
pubmed.ncbi.nlm.nih.gov/17027231/
nature.com/articles/s4138…
pmc.ncbi.nlm.nih.gov/articles/PMC11…
pmc.ncbi.nlm.nih.gov/articles/PMC68…
researchgate.net/publication/31…
researchgate.net/publication/29…
researchgate.net/publication/24…
pmc.ncbi.nlm.nih.gov/articles/PMC10…
pubmed.ncbi.nlm.nih.gov/17027231/
nature.com/articles/s4138…
nature.com/articles/nm.38…
journals.asm.org/doi/10.1128/mb…
journals.plos.org/plospathogens/…
frontiersin.org/journals/micro…
frontiersin.org/journals/endoc…
sciencedirect.com/science/articl…
pmc.ncbi.nlm.nih.gov/articles/PMC61…
pubmed.ncbi.nlm.nih.gov/32418527/
pmc.ncbi.nlm.nih.gov/articles/PMC78…
pubmed.ncbi.nlm.nih.gov/19095412/
journals.asm.org/doi/10.1128/mb…
journals.plos.org/plospathogens/…
frontiersin.org/journals/micro…
frontiersin.org/journals/endoc…
sciencedirect.com/science/articl…
pmc.ncbi.nlm.nih.gov/articles/PMC61…
pubmed.ncbi.nlm.nih.gov/32418527/
pmc.ncbi.nlm.nih.gov/articles/PMC78…
pubmed.ncbi.nlm.nih.gov/19095412/
pubmed.ncbi.nlm.nih.gov/37994672/
pubmed.ncbi.nlm.nih.gov/32021094/ pubmed.ncbi.nlm.nih.gov/27021328/
pubmed.ncbi.nlm.nih.gov/23257726/
pubmed.ncbi.nlm.nih.gov/31334617/
pubmed.ncbi.nlm.nih.gov/33915040/
pubmed.ncbi.nlm.nih.gov/11855736/
pubmed.ncbi.nlm.nih.gov/37043980/
pubmed.ncbi.nlm.nih.gov/27915337/
pubmed.ncbi.nlm.nih.gov/32008756/
pubmed.ncbi.nlm.nih.gov/32021094/ pubmed.ncbi.nlm.nih.gov/27021328/
pubmed.ncbi.nlm.nih.gov/23257726/
pubmed.ncbi.nlm.nih.gov/31334617/
pubmed.ncbi.nlm.nih.gov/33915040/
pubmed.ncbi.nlm.nih.gov/11855736/
pubmed.ncbi.nlm.nih.gov/37043980/
pubmed.ncbi.nlm.nih.gov/27915337/
pubmed.ncbi.nlm.nih.gov/32008756/
• • •
Missing some Tweet in this thread? You can try to
force a refresh