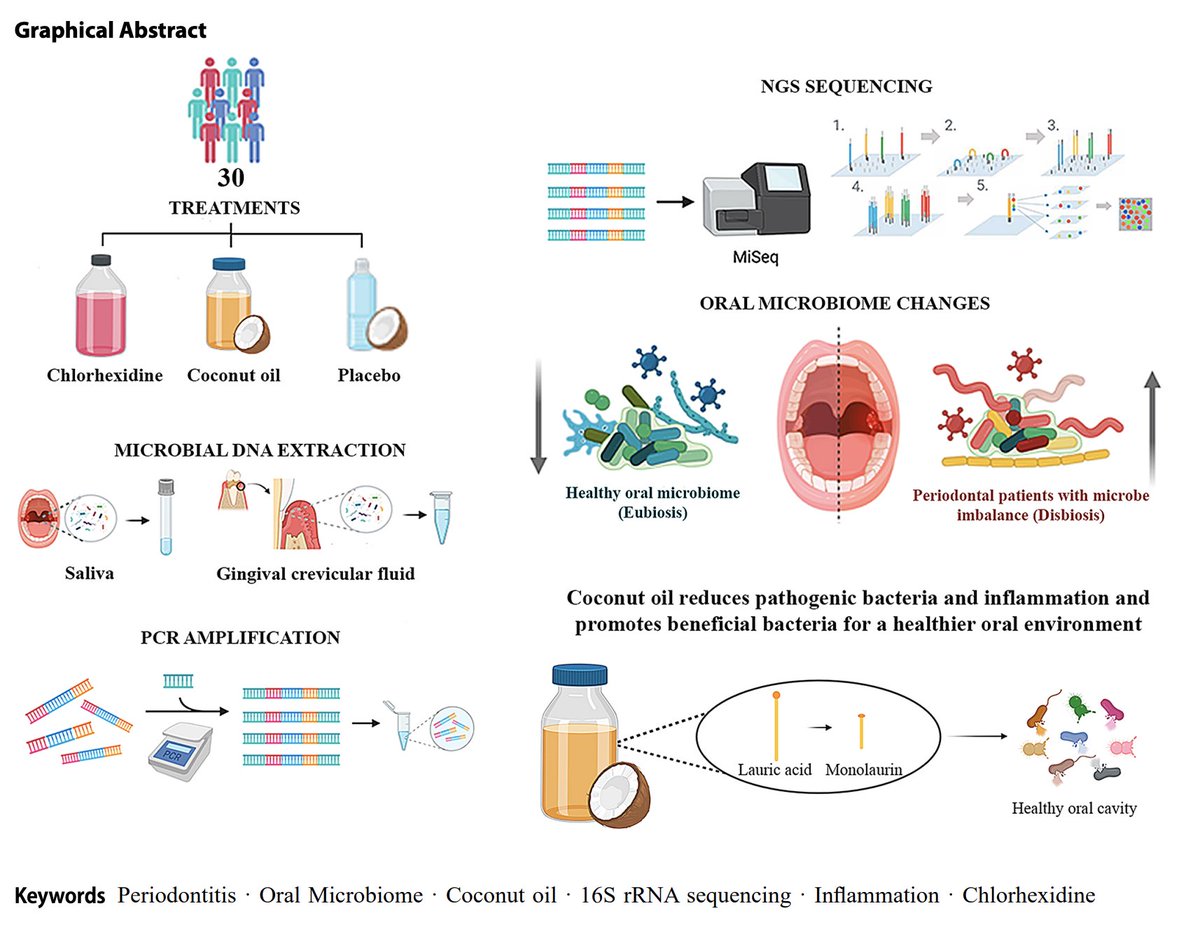
A recent study showed that rinsing with coconut oil was superior to conventional mouthwash for dental health (periodontitis).
(1/8)

(1/8)


The study was published in March in the Journal of Clinical Oral Investigations.
People with periodontitis were given either:
➞ Standard treatment (chlorhexidine mouthwash)
➞ Coconut oil mouthwash (5 mL, 10 mins / day)
➞ Placebo mouthwash
For a month. They were checked in a month after treatment stopped.
(2/8)

People with periodontitis were given either:
➞ Standard treatment (chlorhexidine mouthwash)
➞ Coconut oil mouthwash (5 mL, 10 mins / day)
➞ Placebo mouthwash
For a month. They were checked in a month after treatment stopped.
(2/8)

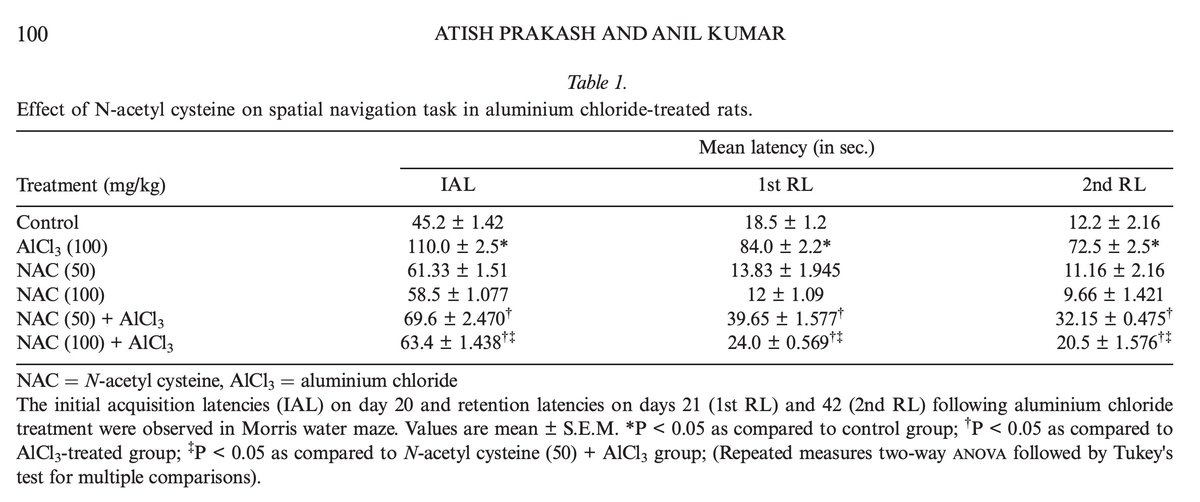
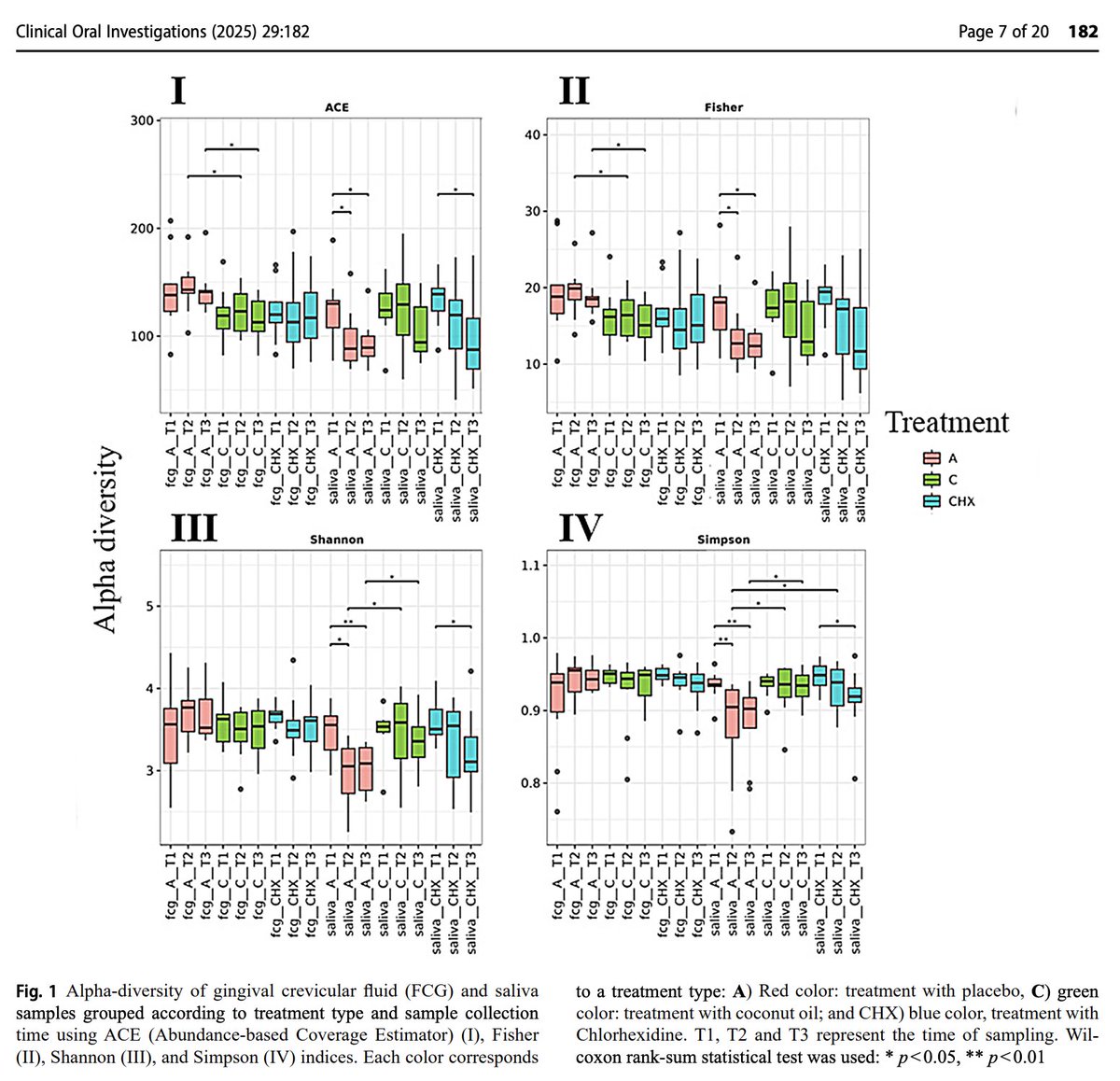
Coconut oil maintained diversity of the oral microbiome.
In both saliva and gingival crevicular fluid, indices of bacterial diversity declined in both placebo and in standard treatment, but not with coconut oil.
This is considered good - as coconut oil did not wipe out bacteria considered beneficial.
(3/8)
In both saliva and gingival crevicular fluid, indices of bacterial diversity declined in both placebo and in standard treatment, but not with coconut oil.
This is considered good - as coconut oil did not wipe out bacteria considered beneficial.
(3/8)
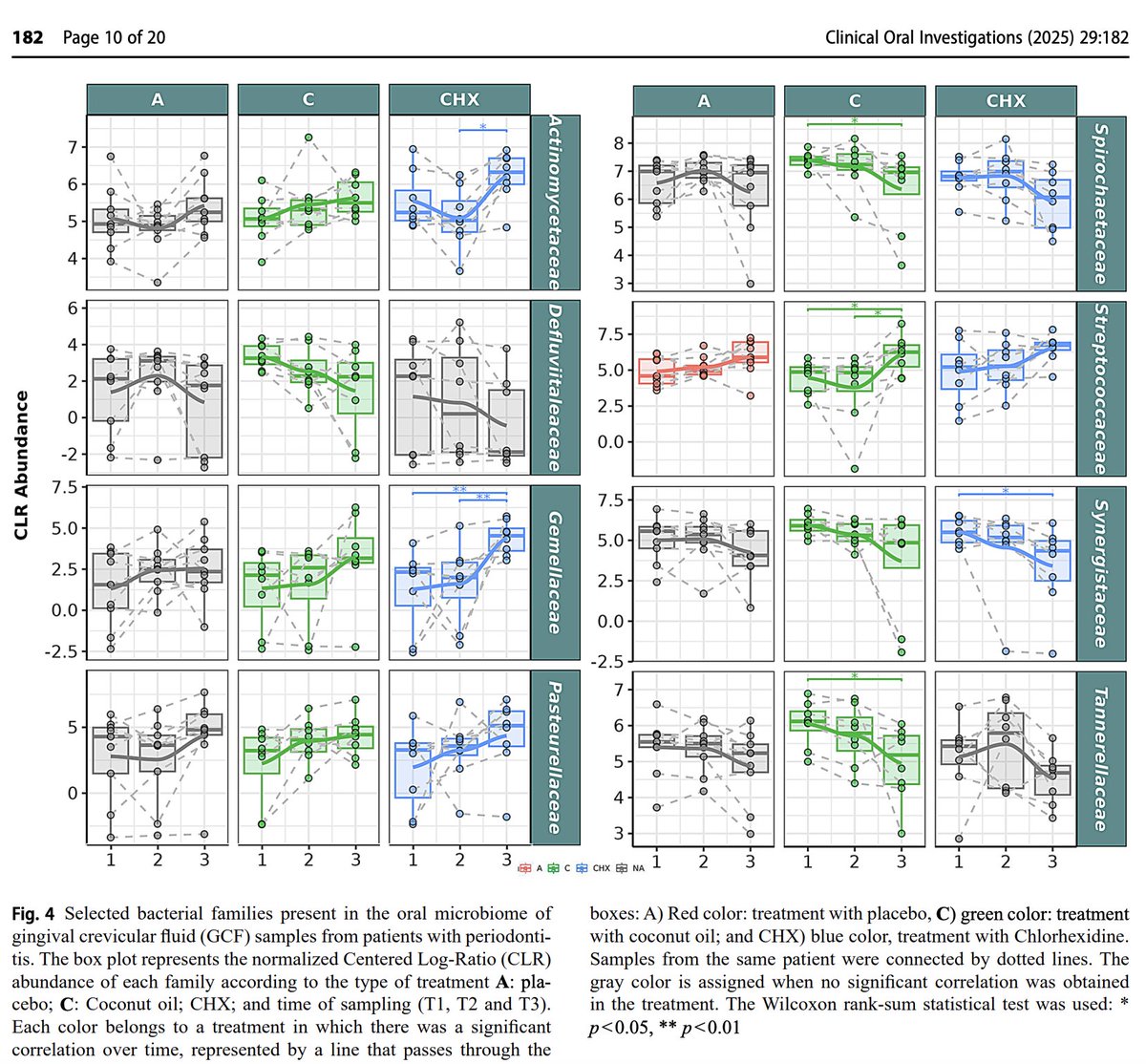
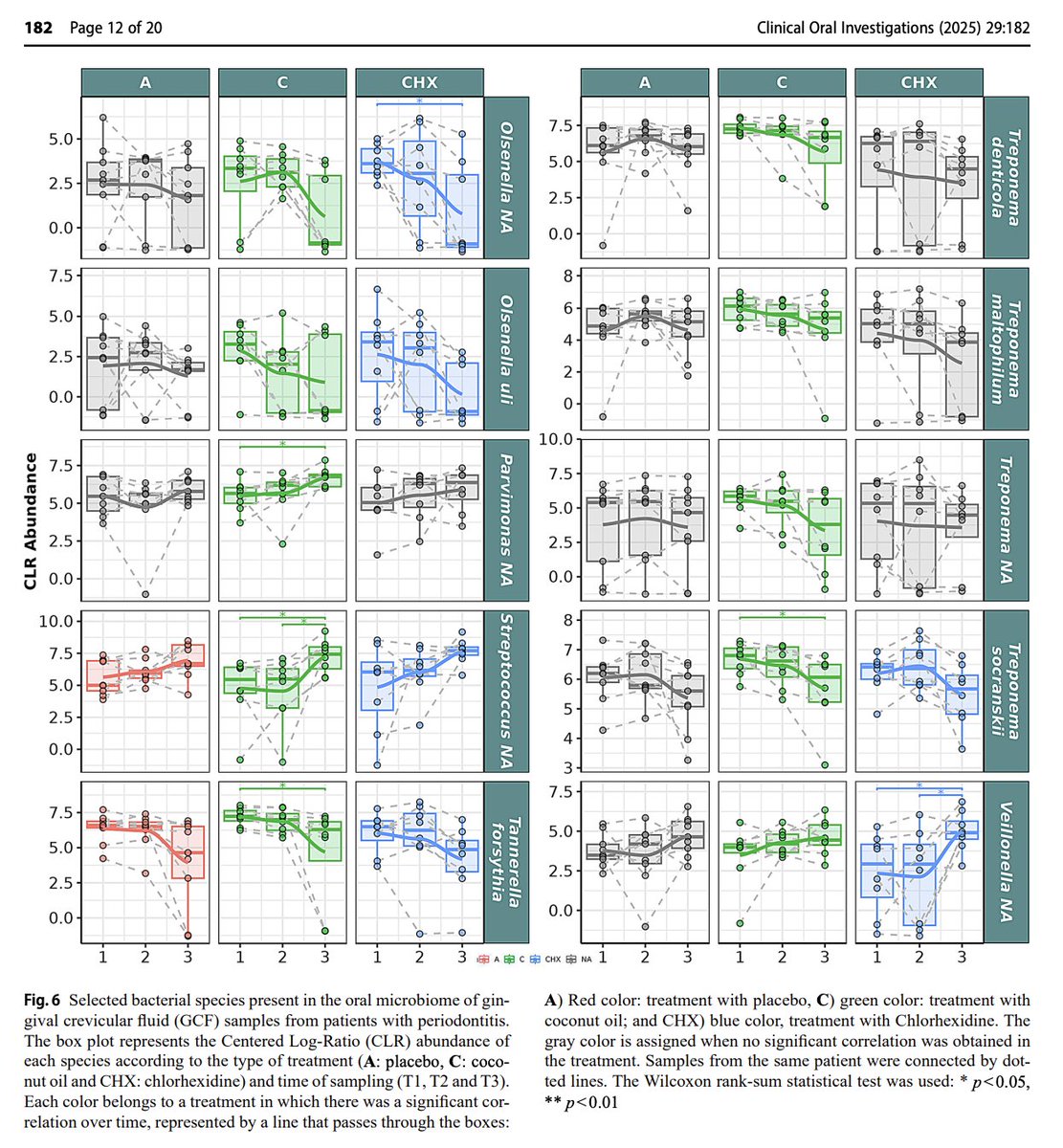
Coconut oil pulling was superior at modifying pathogenic bacteria in the mouth, while increasing commensals.
Pathogens Decreased by Coconut Oil:
↓ Tannerella forsythia (Red complex, major periodontitis pathogen)
↓ Treponema denticola (Red complex, major periodontitis pathogen)
↓ Treponema maltophilum (Anaerobic, disease-associated spirochete)
↓ Treponema socranskii (Periodontitis-associated spirochete)
↓ Treponema (genus level) (Major driver of periodontitis)
↓ Tannerella (Genus includes periodontal pathogens, did not decrease with standard treatment)
↓ Tannerellaceae (Family includes T. forsythia, did not decrease with standard treatment)
↓ Spirochaetaceae (Family of Treponema species, did not decrease with standard treatment)
↓ Synergistaceae (Inflammatory, linked to periodontal pockets)
↓ Olsenella (Pro-inflammatory, found in infected pulp)
↓ Olsenella uli (Pro-inflammatory species)
↓ Fretibacterium (Emerging periodontitis pathogen)
↓ Defluviitaleaceae (Potential pathobiont, poorly understood, did not decrease with standard treatment)
↓ Eubacterium group (Some species linked to disease)
↓ Prevotella (Periodontitis-associated genus)
↓ Prevotella melaninogenica (Inflammation-linked species)
↓ Phocaeicola (Reclassified Bacteroides, inflammatory)
↓ Phocaeicola abscessus (Emerging pathogen)
↓ Bacteroidales incertae sedis (Unclassified, but associated with inflammation)
↓ Campylobacteraceae (Inflammatory, contains pathogenic species)
↓ Granulicatella (Opportunistic, linked to infective endocarditis)
↓ Granulicatella NA (Unclassified strain in this genus)
Good bacteria increased by coconut oil (but NOT by conventional treatment):
↑ Streptococcaceae (Includes protective oral Streptococcus species)
↑ Streptococcus (Protective, anti-inflammatory genus)
↑ Streptococcus mitis (Specific protective species)
↑ Streptococcus NA (Presumed commensal strain)
↑ Gemellaceae (Commensal, protective, early colonizer)
↑ Gemella (Early colonizer, healthy biofilm component)
↑ Pasteurellaceae (Some commensals like Haemophilus)
↑ Actinomycetaceae (Beneficial, early colonizer)
↑ Actinomyces (Anti-inflammatory, early colonizer)
↑ Lachnospiraceae (Butyrate-producing, associated with oral and gut health)
↑ Veillonella (Lactate-utilizing, sometimes protective)
↑ Parvimonas (Mixed data; often in health, also found in disease)
↑ Streptococcus NA (Presumed commensal)
Unclear/Mixed Role or Unexpected:
↑ Oribacterium NA (Increased by CO, unclear role — sometimes linked to inflammation)
↑ Tannerella NA (Unclassified species in a usually pathogenic genus — increase is unexpected)
↓ Carnobacteriaceae (Rare in oral cavity, role unclear)
↓ Oribacterium (Decreased at genus level, conflicting direction vs species level)
Across the board reducing bad guys and increasing good guys.
(4/8)



Pathogens Decreased by Coconut Oil:
↓ Tannerella forsythia (Red complex, major periodontitis pathogen)
↓ Treponema denticola (Red complex, major periodontitis pathogen)
↓ Treponema maltophilum (Anaerobic, disease-associated spirochete)
↓ Treponema socranskii (Periodontitis-associated spirochete)
↓ Treponema (genus level) (Major driver of periodontitis)
↓ Tannerella (Genus includes periodontal pathogens, did not decrease with standard treatment)
↓ Tannerellaceae (Family includes T. forsythia, did not decrease with standard treatment)
↓ Spirochaetaceae (Family of Treponema species, did not decrease with standard treatment)
↓ Synergistaceae (Inflammatory, linked to periodontal pockets)
↓ Olsenella (Pro-inflammatory, found in infected pulp)
↓ Olsenella uli (Pro-inflammatory species)
↓ Fretibacterium (Emerging periodontitis pathogen)
↓ Defluviitaleaceae (Potential pathobiont, poorly understood, did not decrease with standard treatment)
↓ Eubacterium group (Some species linked to disease)
↓ Prevotella (Periodontitis-associated genus)
↓ Prevotella melaninogenica (Inflammation-linked species)
↓ Phocaeicola (Reclassified Bacteroides, inflammatory)
↓ Phocaeicola abscessus (Emerging pathogen)
↓ Bacteroidales incertae sedis (Unclassified, but associated with inflammation)
↓ Campylobacteraceae (Inflammatory, contains pathogenic species)
↓ Granulicatella (Opportunistic, linked to infective endocarditis)
↓ Granulicatella NA (Unclassified strain in this genus)
Good bacteria increased by coconut oil (but NOT by conventional treatment):
↑ Streptococcaceae (Includes protective oral Streptococcus species)
↑ Streptococcus (Protective, anti-inflammatory genus)
↑ Streptococcus mitis (Specific protective species)
↑ Streptococcus NA (Presumed commensal strain)
↑ Gemellaceae (Commensal, protective, early colonizer)
↑ Gemella (Early colonizer, healthy biofilm component)
↑ Pasteurellaceae (Some commensals like Haemophilus)
↑ Actinomycetaceae (Beneficial, early colonizer)
↑ Actinomyces (Anti-inflammatory, early colonizer)
↑ Lachnospiraceae (Butyrate-producing, associated with oral and gut health)
↑ Veillonella (Lactate-utilizing, sometimes protective)
↑ Parvimonas (Mixed data; often in health, also found in disease)
↑ Streptococcus NA (Presumed commensal)
Unclear/Mixed Role or Unexpected:
↑ Oribacterium NA (Increased by CO, unclear role — sometimes linked to inflammation)
↑ Tannerella NA (Unclassified species in a usually pathogenic genus — increase is unexpected)
↓ Carnobacteriaceae (Rare in oral cavity, role unclear)
↓ Oribacterium (Decreased at genus level, conflicting direction vs species level)
Across the board reducing bad guys and increasing good guys.
(4/8)


Coconut oil also markedly improved the subgingival dysbiosis index,
a calculation of overall bacterial health.
(5/8)
a calculation of overall bacterial health.
(5/8)

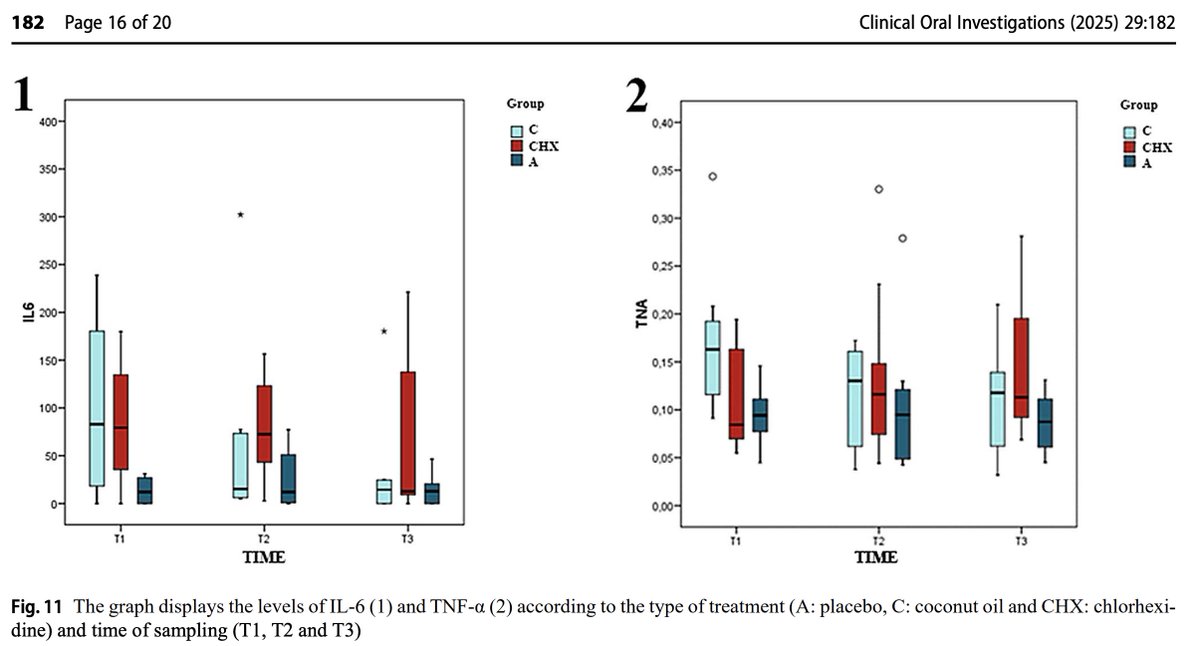
Coconut oil also had a powerful anti-inflammatory effect.
It lowered:
◇ TNFα
◇ IL-6
While other treatments did not.
(6/8)
It lowered:
◇ TNFα
◇ IL-6
While other treatments did not.
(6/8)
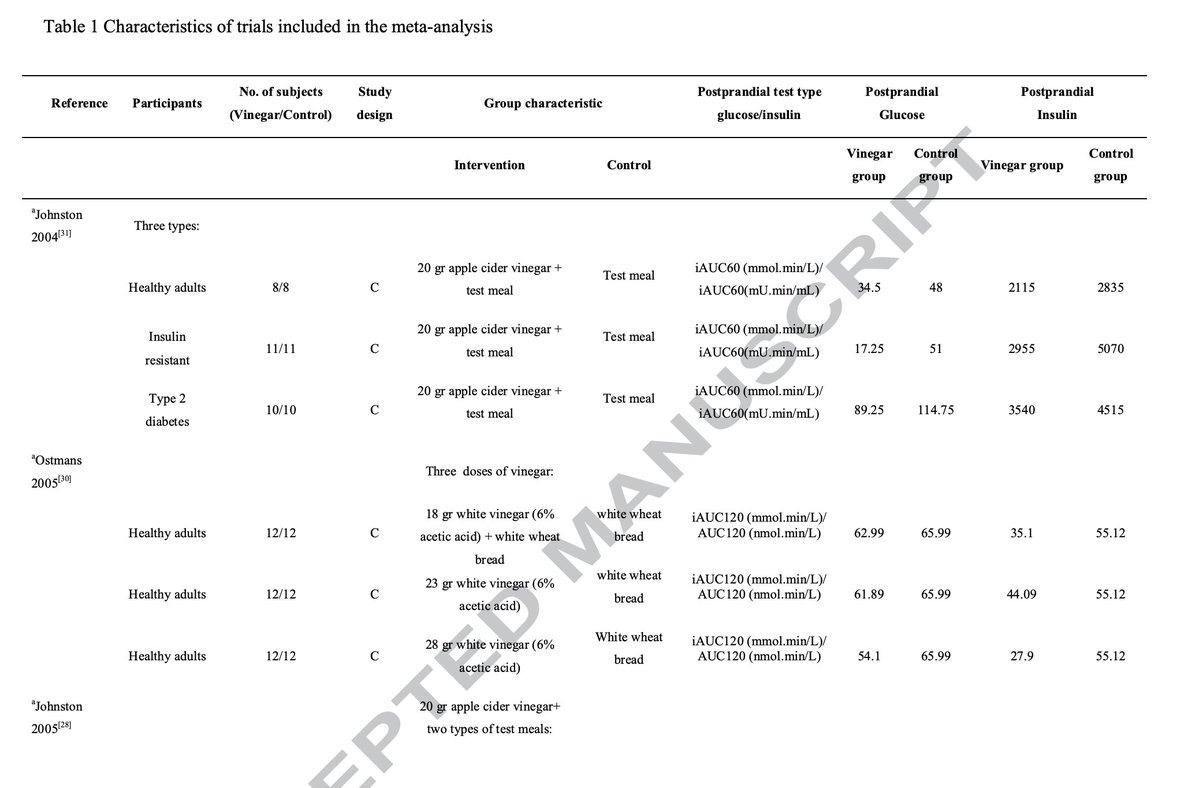
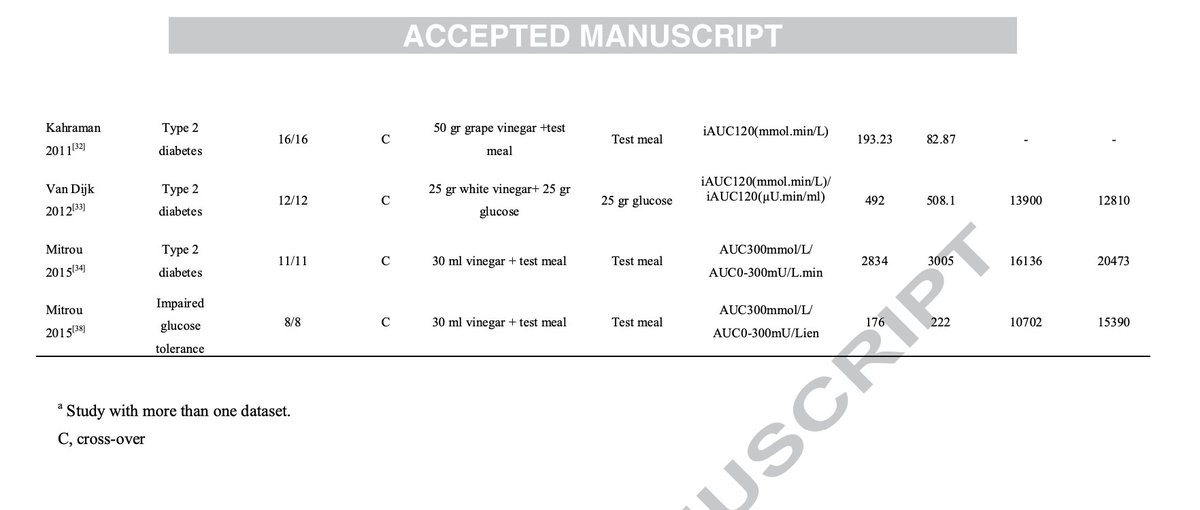
Multiple other studies have also shown that coconut oil pulling is an excellent tool for improving dental health, reducing plaques and cavity risk.
(7/8)

(7/8)


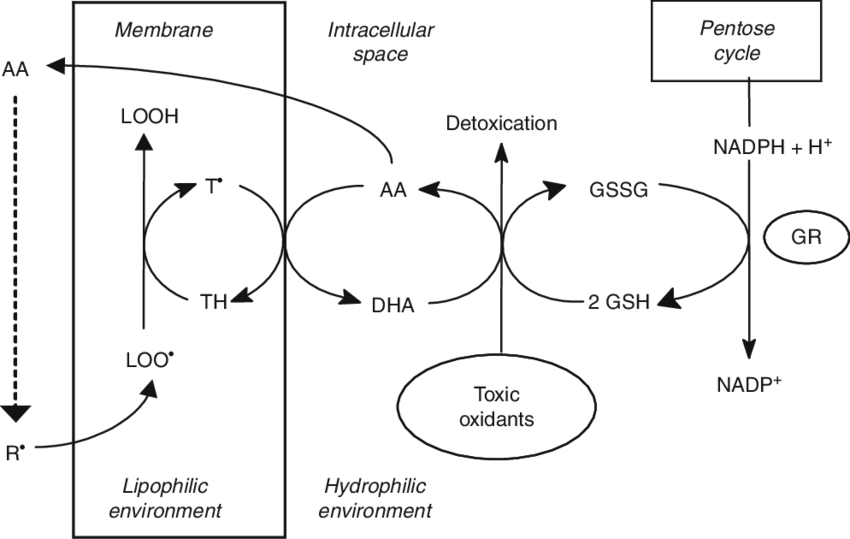
HOW coconut oil works for dental health:
The magic here is in its medium chain fats:
Lauric acid (C12:0)
Capric acid (C10:0)
Caprylic acid (C8:0)
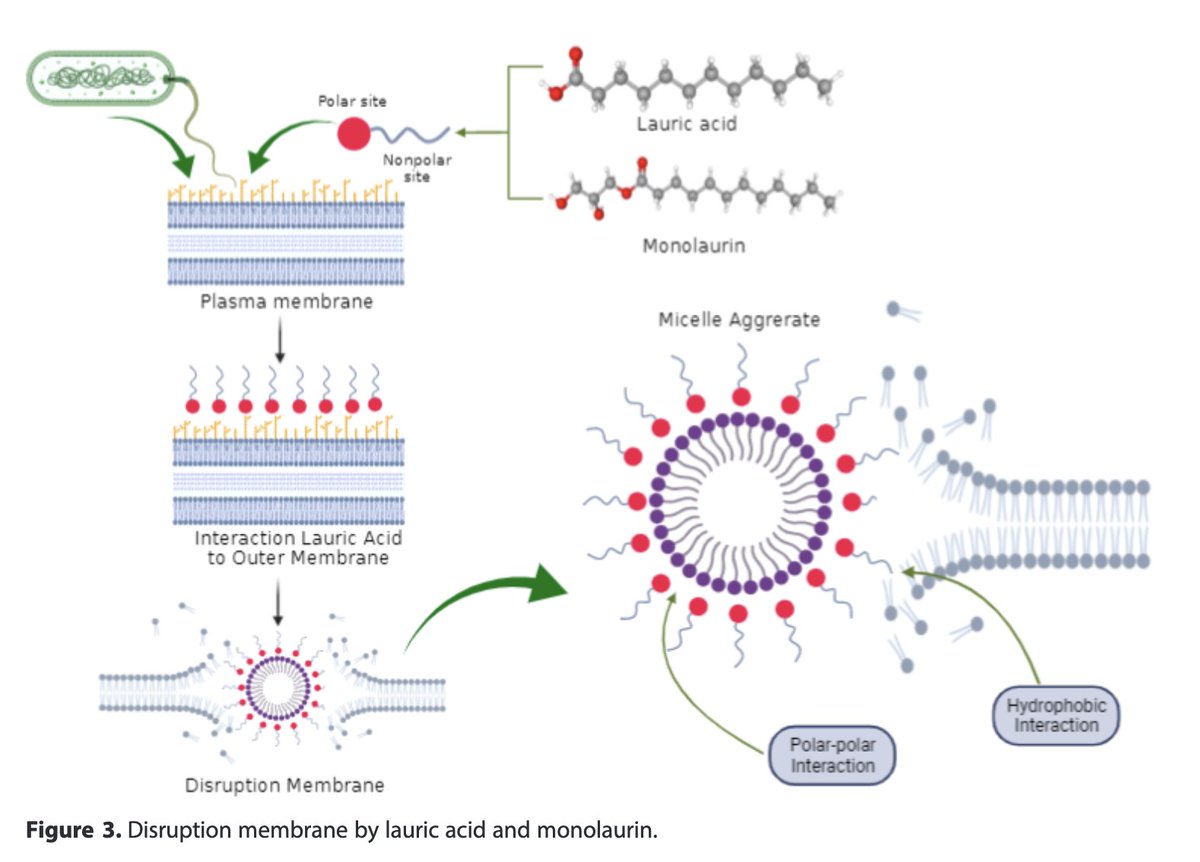
Lauric acid (monolaurin) disrupts bacterial membranes, particularly those of gram-negative and lipid-enveloped bacteria, both of which tend to be pathogenic bacteria.
However, commensal or good bacteria tend to be spared from these effects, unlike broad antimicrobials like antibiotics or the standard chlorhexidine treatment.
Thus, it positively modulates the oral bacteria, which lowers inflammation in the mouth.
(8/8)
The magic here is in its medium chain fats:
Lauric acid (C12:0)
Capric acid (C10:0)
Caprylic acid (C8:0)
Lauric acid (monolaurin) disrupts bacterial membranes, particularly those of gram-negative and lipid-enveloped bacteria, both of which tend to be pathogenic bacteria.
However, commensal or good bacteria tend to be spared from these effects, unlike broad antimicrobials like antibiotics or the standard chlorhexidine treatment.
Thus, it positively modulates the oral bacteria, which lowers inflammation in the mouth.
(8/8)
If YOU want PERSONALIZED, IN DEPTH help from us with any of your health goals, schedule a free call here: go.prism.miami/consultation
Read the full study here: pmc.ncbi.nlm.nih.gov/articles/PMC11…
• • •
Missing some Tweet in this thread? You can try to
force a refresh