🧵 Drug Combinations That Can Kill — Interactions You Must Never Miss
We prescribe these daily.
Get the combination wrong → bleeding, rhabdomyolysis, bone marrow suppression, cardiac arrest.
Here are the 10 combinations you must always check for 👇
@DrAkhilX @IhabFathiSulima @CelestinoGutirr @Janetbirdope @DurgaPrasannaM1 @SarahSchaferMD @NeuroSjogrens #MedTwitter #RheumTwitter
We prescribe these daily.
Get the combination wrong → bleeding, rhabdomyolysis, bone marrow suppression, cardiac arrest.
Here are the 10 combinations you must always check for 👇
@DrAkhilX @IhabFathiSulima @CelestinoGutirr @Janetbirdope @DurgaPrasannaM1 @SarahSchaferMD @NeuroSjogrens #MedTwitter #RheumTwitter
1) Allopurinol or Febuxostat + Azathioprine or 6-Mercaptopurine
❌ Severe bone marrow suppression (xanthine oxidase inhibition).
✅ Avoid the combination; if unavoidable, drastically reduce azathioprine dose and monitor blood counts closely — but switching is safer.
❌ Severe bone marrow suppression (xanthine oxidase inhibition).
✅ Avoid the combination; if unavoidable, drastically reduce azathioprine dose and monitor blood counts closely — but switching is safer.

2) Methotrexate + Trimethoprim–Sulfamethoxazole (Co-trimoxazole)
❌ Pancytopenia, mucositis, acute kidney injury.
✅ Use alternatives such as nitrofurantoin or fosfomycin for urinary tract infections.
❌ Pancytopenia, mucositis, acute kidney injury.
✅ Use alternatives such as nitrofurantoin or fosfomycin for urinary tract infections.

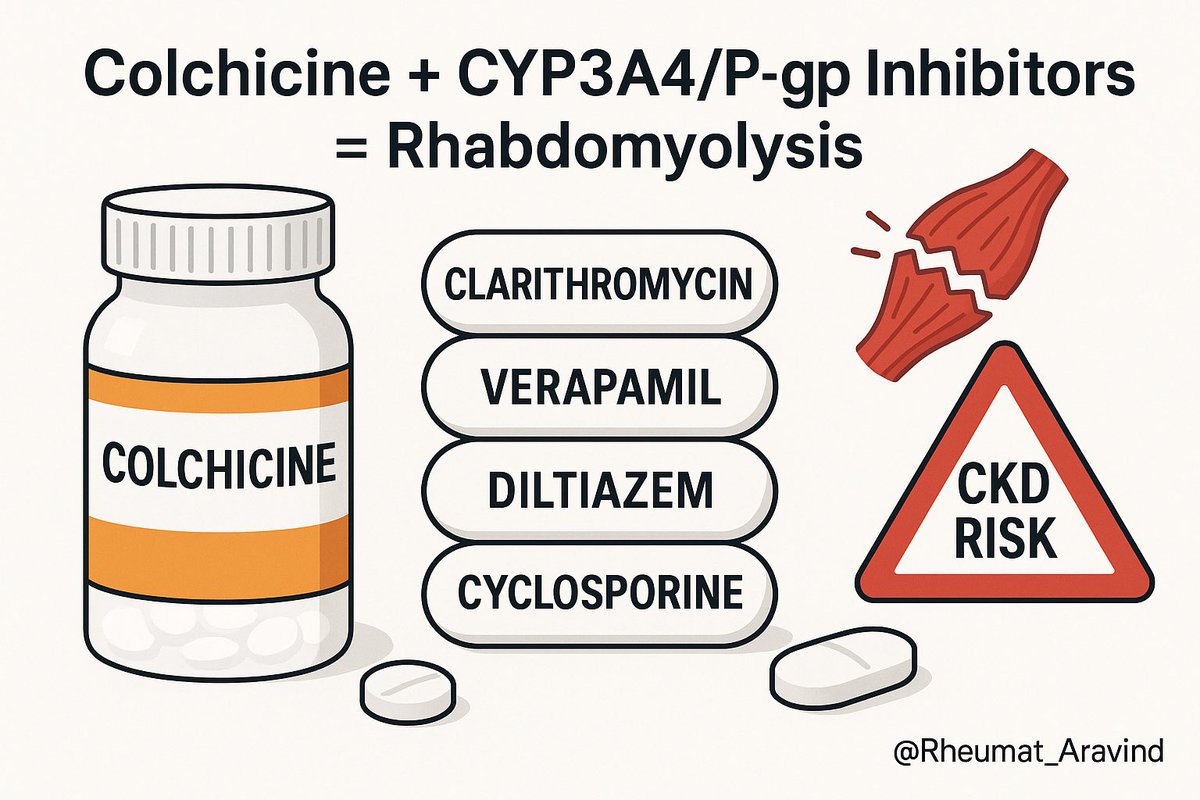
3) Colchicine + CYP3A4 or P-glycoprotein inhibitors (e.g., clarithromycin, verapamil, diltiazem, cyclosporine), especially in chronic kidney disease
❌ Toxicity and rhabdomyolysis, possible multi-organ failure.
✅ Prefer azithromycin; avoid combination or reduce colchicine dose significantly.
❌ Toxicity and rhabdomyolysis, possible multi-organ failure.
✅ Prefer azithromycin; avoid combination or reduce colchicine dose significantly.
4) Simvastatin or Lovastatin + Strong CYP3A4 inhibitors (e.g., clarithromycin, ketoconazole, protease inhibitors)
❌ Rhabdomyolysis.
✅ Switch to pravastatin or rosuvastatin, or change the interacting drug.
❌ Rhabdomyolysis.
✅ Switch to pravastatin or rosuvastatin, or change the interacting drug.

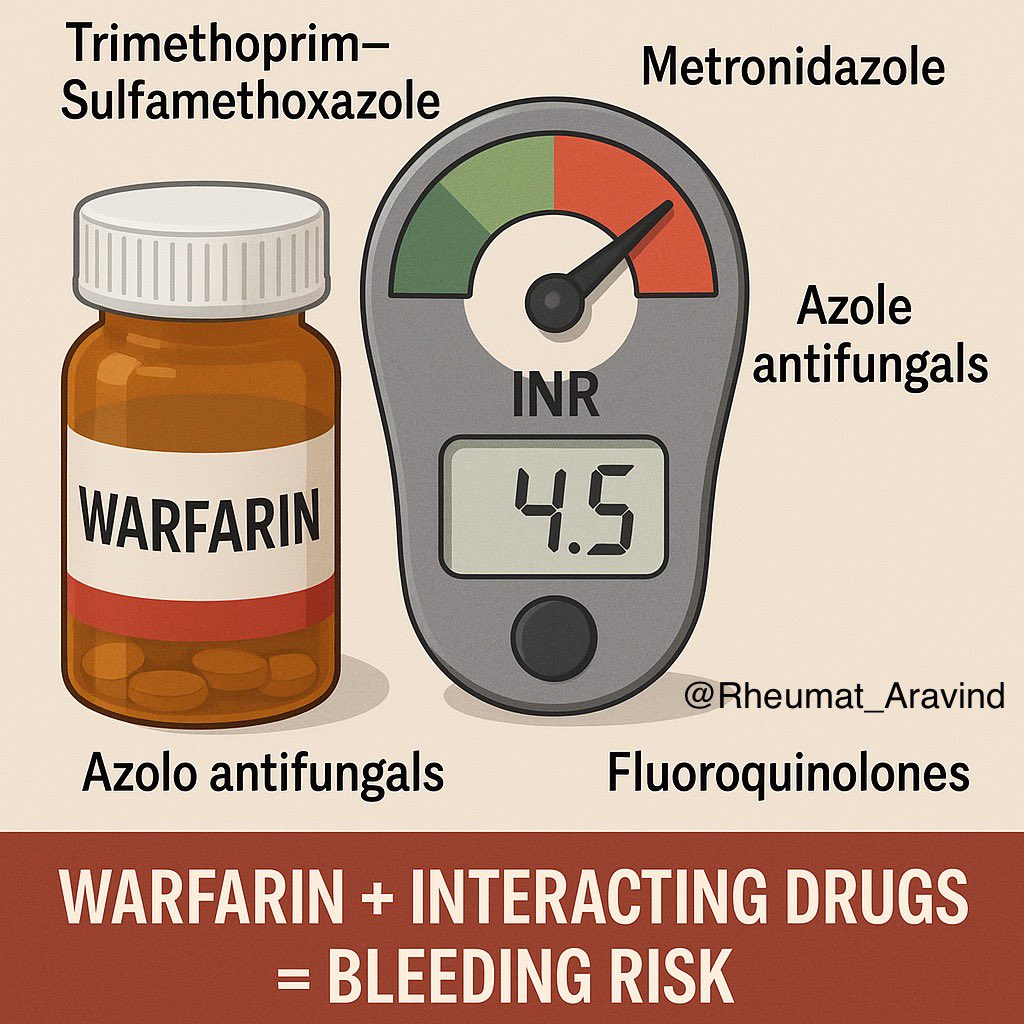
5) Warfarin + Trimethoprim–Sulfamethoxazole, Metronidazole, Azole antifungals, or Fluoroquinolones
❌ International Normalized Ratio (INR) spike → major bleeding.
✅ Reduce warfarin dose pre-emptively and monitor INR early, or choose a safer alternative.
❌ International Normalized Ratio (INR) spike → major bleeding.
✅ Reduce warfarin dose pre-emptively and monitor INR early, or choose a safer alternative.
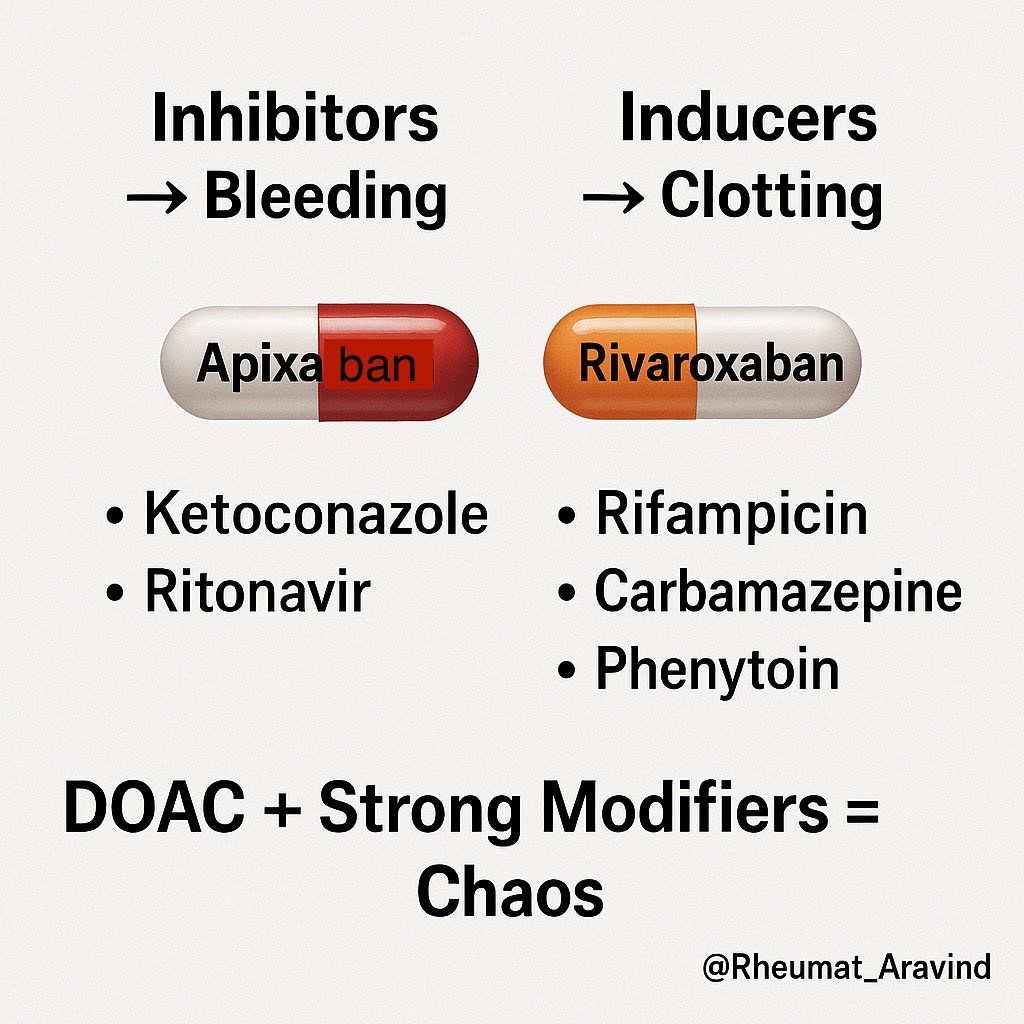
6) Apixaban or Rivaroxaban + Strong CYP3A4 or P-glycoprotein inhibitors or inducers (e.g., ketoconazole, ritonavir, rifampicin, carbamazepine, phenytoin)
❌ Bleeding (with inhibitors) or clotting (with inducers).
✅ Avoid combination; change anticoagulant or interacting drug.
❌ Bleeding (with inhibitors) or clotting (with inducers).
✅ Avoid combination; change anticoagulant or interacting drug.
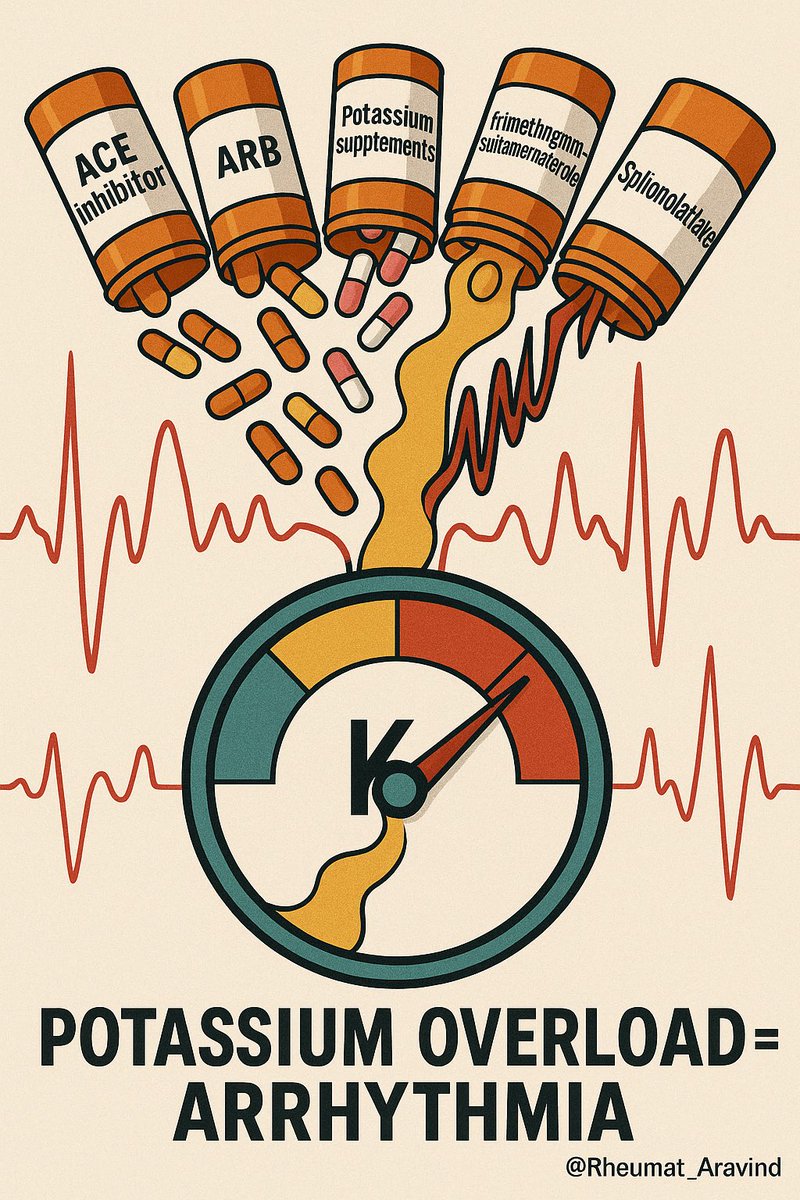
7) Angiotensin-converting enzyme inhibitor or Angiotensin receptor blocker + Spironolactone + Potassium supplements ± Trimethoprim–Sulfamethoxazole
❌ Dangerous hyperkalemia → arrhythmia (especially in chronic kidney disease or elderly).
✅ Avoid stacking; monitor potassium and creatinine closely; use non-trimethoprim antibiotics if possible.
❌ Dangerous hyperkalemia → arrhythmia (especially in chronic kidney disease or elderly).
✅ Avoid stacking; monitor potassium and creatinine closely; use non-trimethoprim antibiotics if possible.
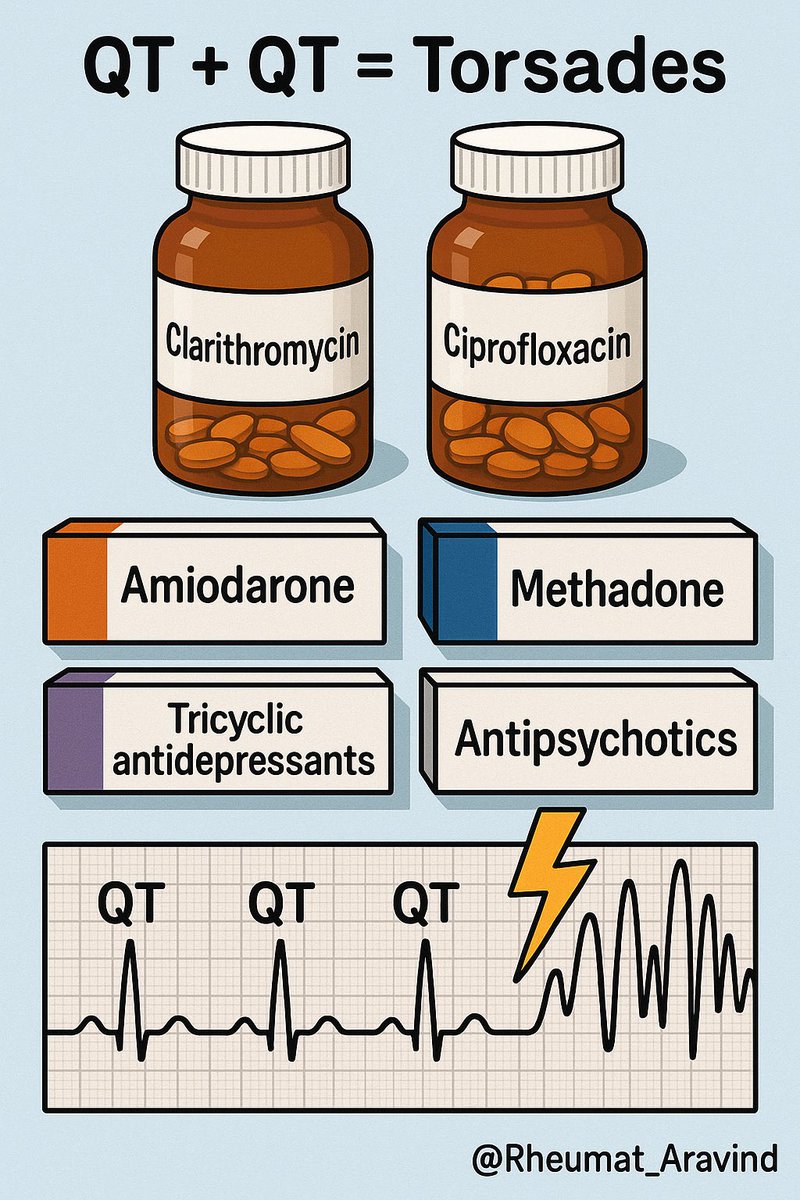
8) Macrolide antibiotics or Fluoroquinolone antibiotics + Other QT-prolonging drugs (e.g., amiodarone, antipsychotics, methadone, tricyclic antidepressants)
❌ Torsades de pointes.
✅ Baseline ECG; avoid combination; consider doxycycline where appropriate.
❌ Torsades de pointes.
✅ Baseline ECG; avoid combination; consider doxycycline where appropriate.
9) Linezolid + Selective serotonin reuptake inhibitors (SSRIs), Serotonin–norepinephrine reuptake inhibitors (SNRIs), Monoamine oxidase inhibitors (MAOIs), or Triptans
❌ Serotonin syndrome.
✅ Hold serotonergic drugs if possible and monitor closely.
❌ Serotonin syndrome.
✅ Hold serotonergic drugs if possible and monitor closely.

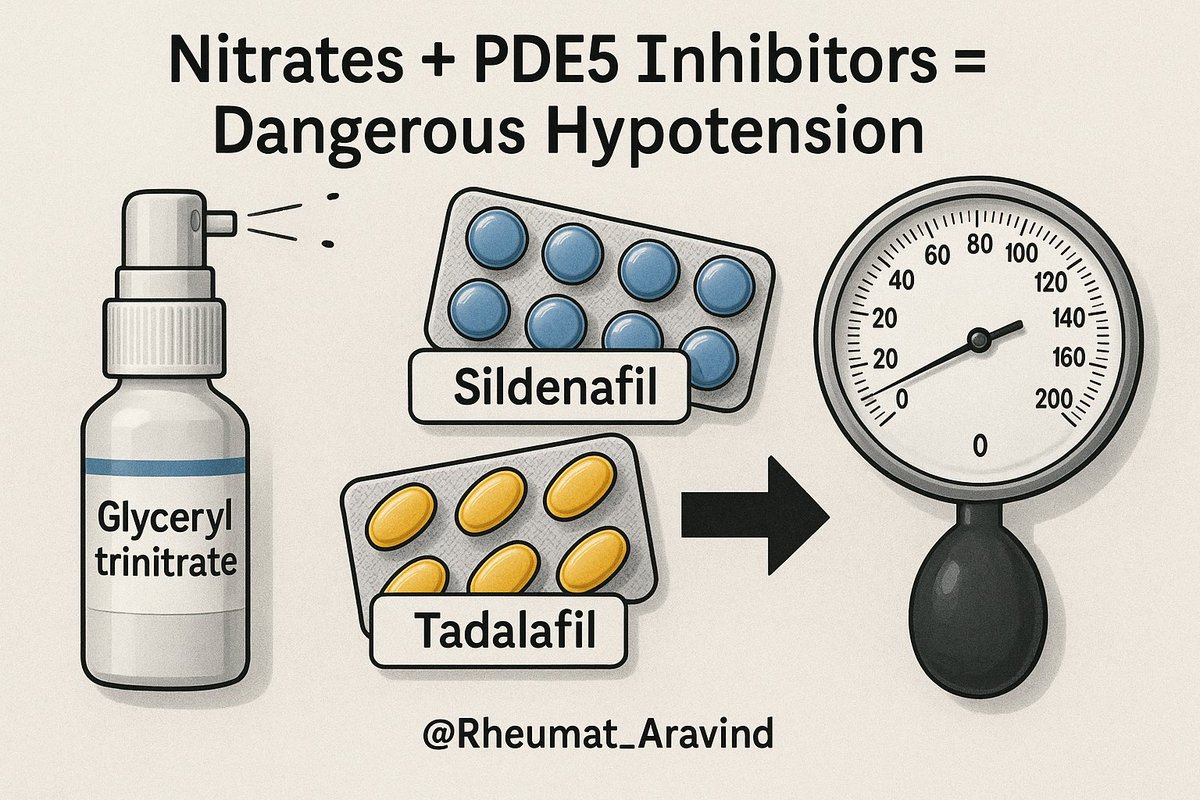
10) Nitrates (e.g., glyceryl trinitrate) + Phosphodiesterase-5 inhibitors (e.g., sildenafil, tadalafil)
❌ Profound hypotension.
✅ Never co-administer; observe washout periods of at least 24–48 hours depending on the drug.
❌ Profound hypotension.
✅ Never co-administer; observe washout periods of at least 24–48 hours depending on the drug.
📌 Takeaway
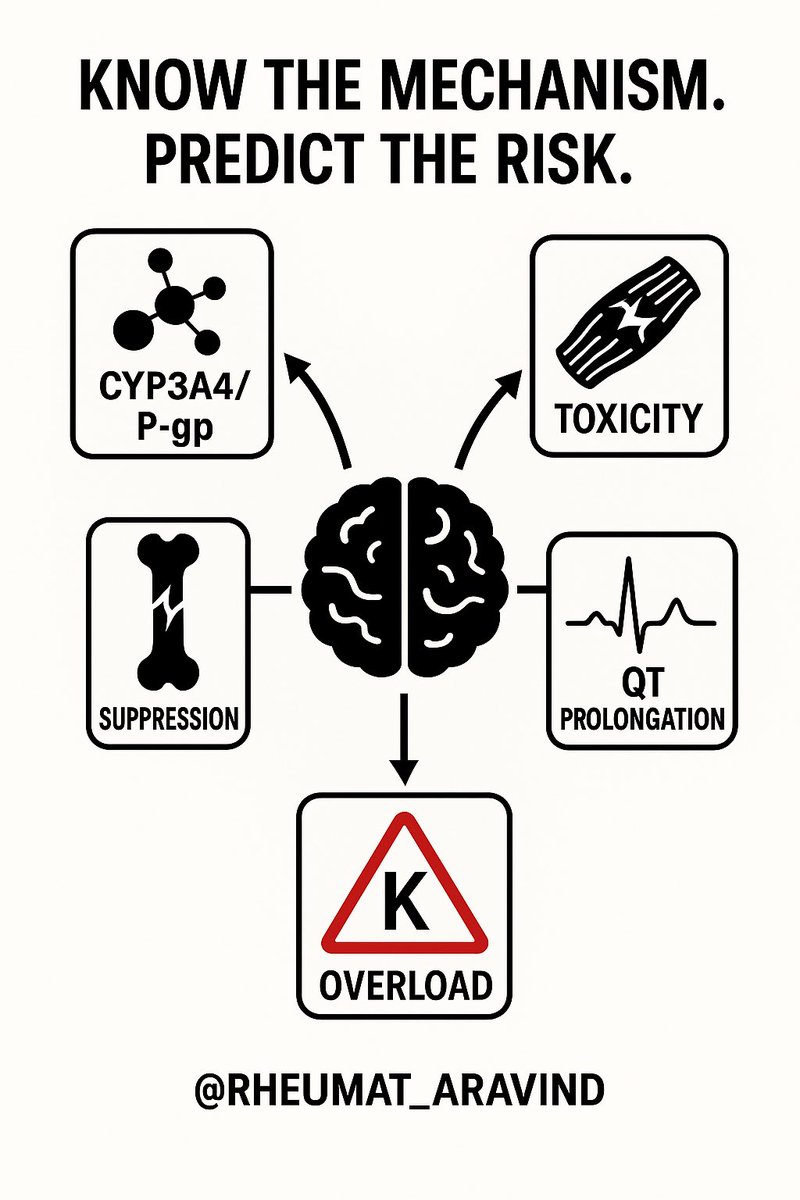
Most interactions are predictable if you know the mechanism.
Look out for:
– CYP3A4 and P-glycoprotein effects
– Bone marrow suppression
– Muscle toxicity
– QT prolongation
– Potassium overload
💬 Share this — it could prevent a catastrophe in your next prescription.
Most interactions are predictable if you know the mechanism.
Look out for:
– CYP3A4 and P-glycoprotein effects
– Bone marrow suppression
– Muscle toxicity
– QT prolongation
– Potassium overload
💬 Share this — it could prevent a catastrophe in your next prescription.
• • •
Missing some Tweet in this thread? You can try to
force a refresh